Abstract
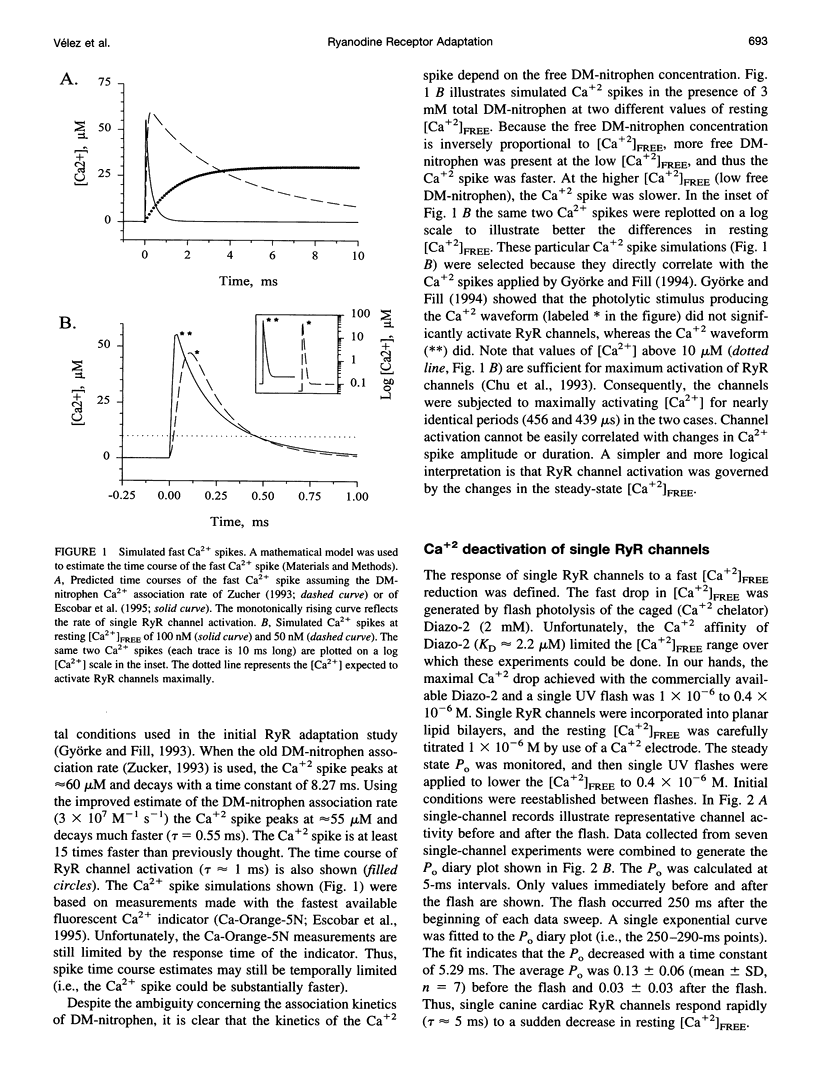
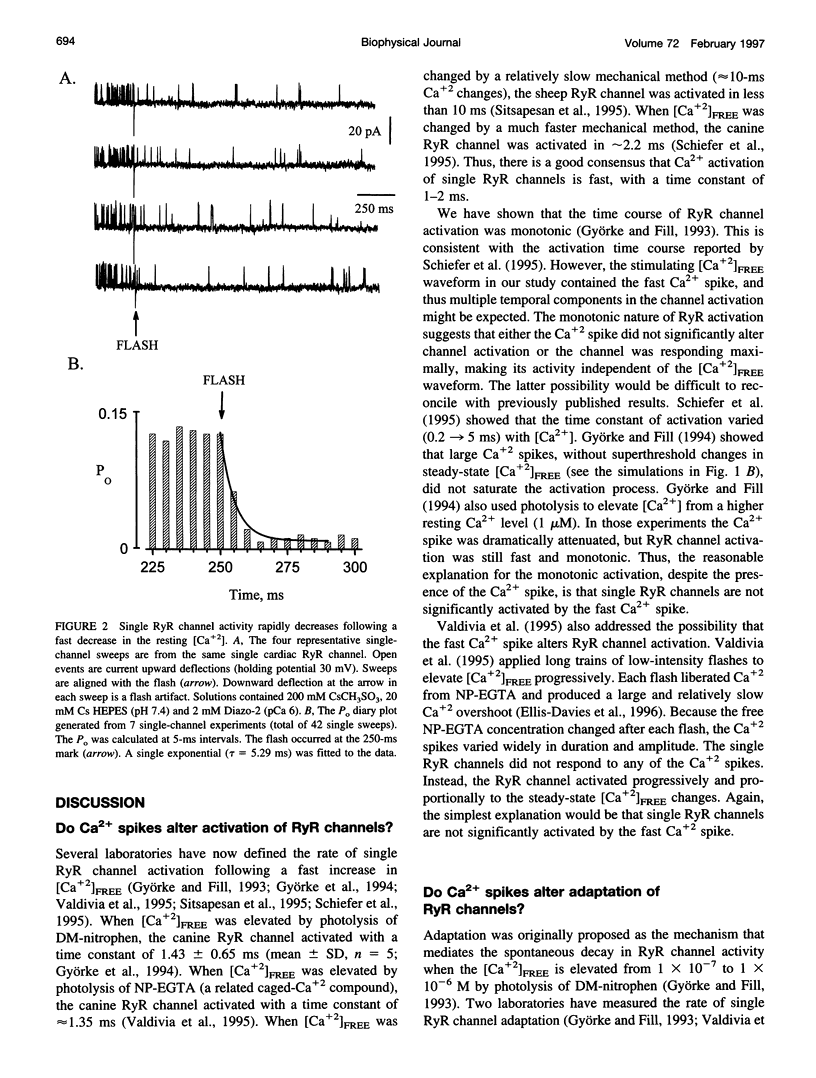
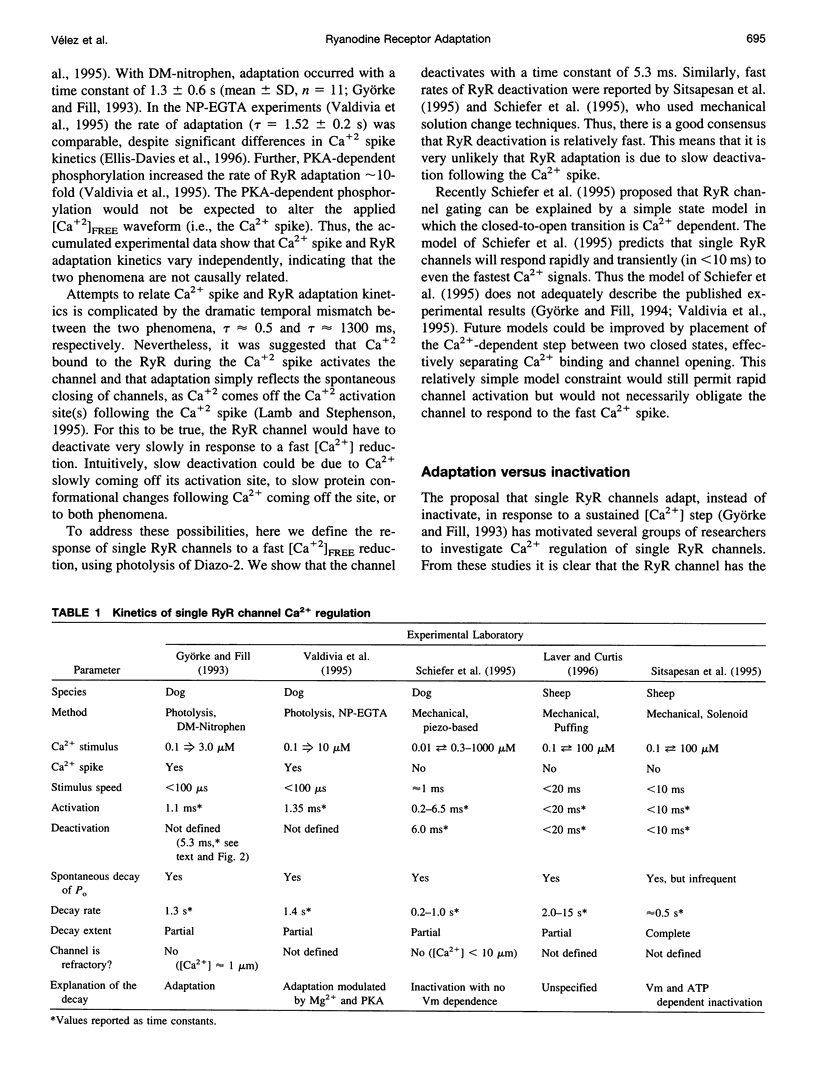
Single cardiac ryanodine receptor (RyR) channel adaptation was previously defined with Ca2+ stimuli produced by flash photolysis of DM-nitrophen (caged-Ca+2). Photolysis of DM-nitrophen induced a very fast Ca+2 overshoot (Ca+2 spike) at the leading edge of the Ca+2 stimuli. It has been suggested that adaptation (τ ≈ 1.3 s) may reflect Ca+2 slowly coming off the RyR Ca+2 activation sites following the faster Ca+2 spike (τ ≈ 1 ms). This concern was addressed by defining the Ca2+ deactivation kinetics of single RyR channels in response to a rapid reduction in free Ca2+ concentration ([Ca2+]FREE). The [Ca2+]FREE was lowered by photolysis of Diazo-2. Single RyR channels deactivated (τ ≈ 5.3 ms) quickly in response to the photolytically induced [Ca2+]FREE reduction. Improved estimates of the Ca2+ spike time course indicate that the Ca2+ spike is considerably faster (10-100-fold) than previously thought. Our data suggest that single RyRs are not significantly activated by fast Ca2+ spikes and that RyR adaptation is not due to deactivation following the fast Ca2+ spike. Thus, RyR adaptation may have an important impact on Ca2+ signaling in heart.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu A., Fill M., Stefani E., Entman M. L. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca(2+)-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol. 1993 Jul;135(1):49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G. C., Kaplan J. H., Barsotti R. J. Laser photolysis of caged calcium: rates of calcium release by nitrophenyl-EGTA and DM-nitrophen. Biophys J. 1996 Feb;70(2):1006–1016. doi: 10.1016/S0006-3495(96)79644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar A. L., Cifuentes F., Vergara J. L. Detection of Ca(2+)-transients elicited by flash photolysis of DM-nitrophen with a fast calcium indicator. FEBS Lett. 1995 May 15;364(3):335–338. doi: 10.1016/0014-5793(95)00425-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke I., Gyorke S. Adaptive control of intracellular Ca2+ release in C2C12 mouse myotubes. Pflugers Arch. 1996 Apr;431(6):838–843. doi: 10.1007/s004240050075. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Response. Science. 1994 Feb 18;263(5149):987–988. doi: 10.1126/science.263.5149.987. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Vélez P., Suárez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J. 1994 Jun;66(6):1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Activation of ryanodine receptors by flash photolysis of caged Ca2+. Biophys J. 1995 Mar;68(3):946–948. doi: 10.1016/S0006-3495(95)80270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R., Dulhunty A. F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995 Sep;147(1):7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Fidler-Lim N., Ellis-Davies G. C., Kaplan J. H. Rate of release of Ca2+ following laser photolysis of the DM-nitrophen-Ca2+ complex. Biochemistry. 1992 Sep 22;31(37):8856–8861. doi: 10.1021/bi00152a023. [DOI] [PubMed] [Google Scholar]
- Mulligan I. P., Ashley C. C. Rapid relaxation of single frog skeletal muscle fibres following laser flash photolysis of the caged calcium chelator, diazo-2. FEBS Lett. 1989 Sep 11;255(1):196–200. doi: 10.1016/0014-5793(89)81090-7. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Morad M. Ca2(+)-induced Ca2+ release as examined by photolysis of caged Ca2+ in single ventricular myocytes. Am J Physiol. 1990 Jan;258(1 Pt 1):C189–C193. doi: 10.1152/ajpcell.1990.258.1.C189. [DOI] [PubMed] [Google Scholar]
- Ríos E. Reining in calcium release. Biophys J. 1994 Jul;67(1):7–9. doi: 10.1016/S0006-3495(94)80451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer A., Meissner G., Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca(2+)-release channels. J Physiol. 1995 Dec 1;489(Pt 2):337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Montgomery R. A., Williams A. J. New insights into the gating mechanisms of cardiac ryanodine receptors revealed by rapid changes in ligand concentration. Circ Res. 1995 Oct;77(4):765–772. doi: 10.1161/01.res.77.4.765. [DOI] [PubMed] [Google Scholar]
- Tate C. A., Bick R. J., Chu A., Van Winkle W. B., Entman M. L. Nucleotide specificity of cardiac sarcoplasmic reticulum. GTP-induced calcium accumulation and GTPase activity. J Biol Chem. 1985 Aug 15;260(17):9618–9623. [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. The calcium concentration clamp: spikes and reversible pulses using the photolabile chelator DM-nitrophen. Cell Calcium. 1993 Feb;14(2):87–100. doi: 10.1016/0143-4160(93)90079-l. [DOI] [PubMed] [Google Scholar]
- Zucker R. Photorelease techniques for raising or lowering intracellular Ca2+. Methods Cell Biol. 1994;40:31–63. doi: 10.1016/s0091-679x(08)61109-7. [DOI] [PubMed] [Google Scholar]


