Abstract
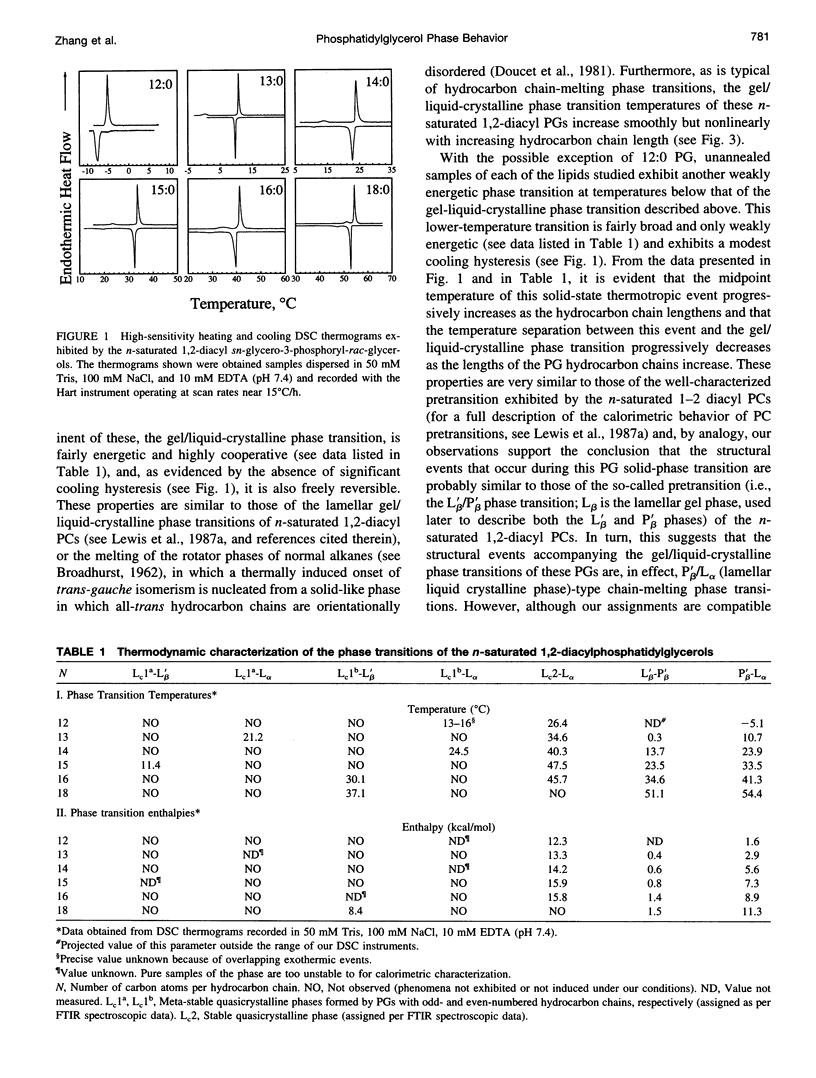
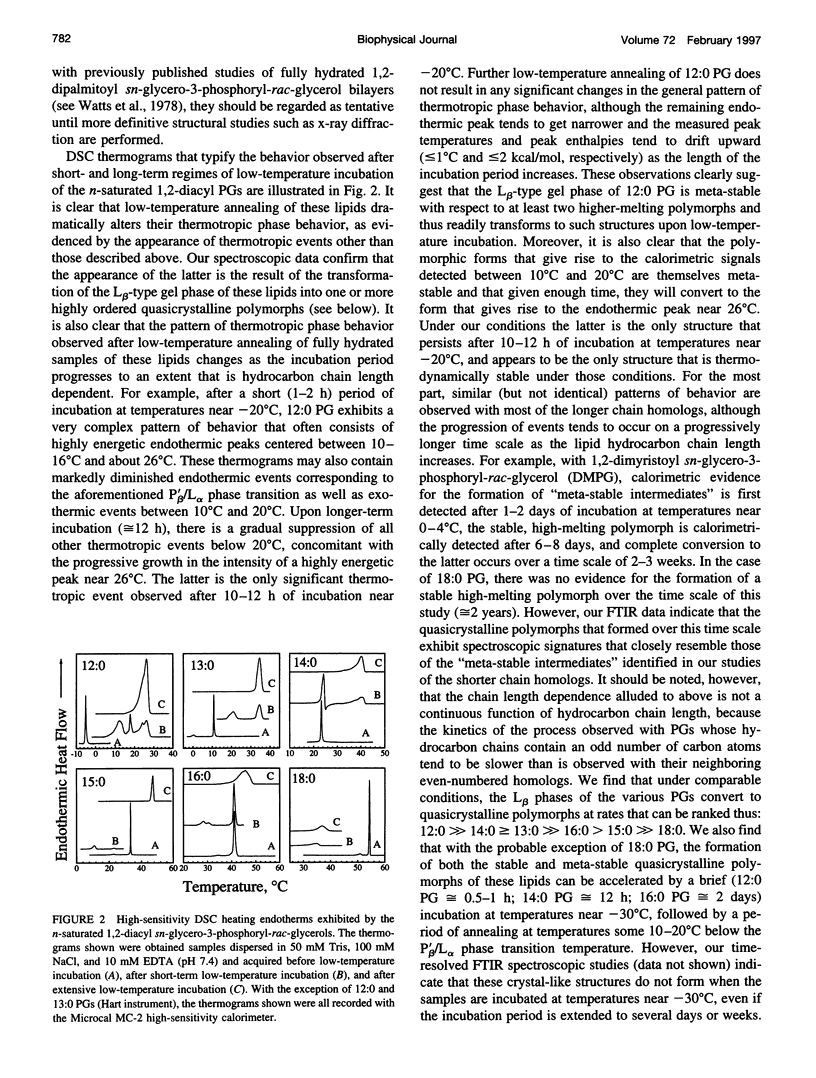
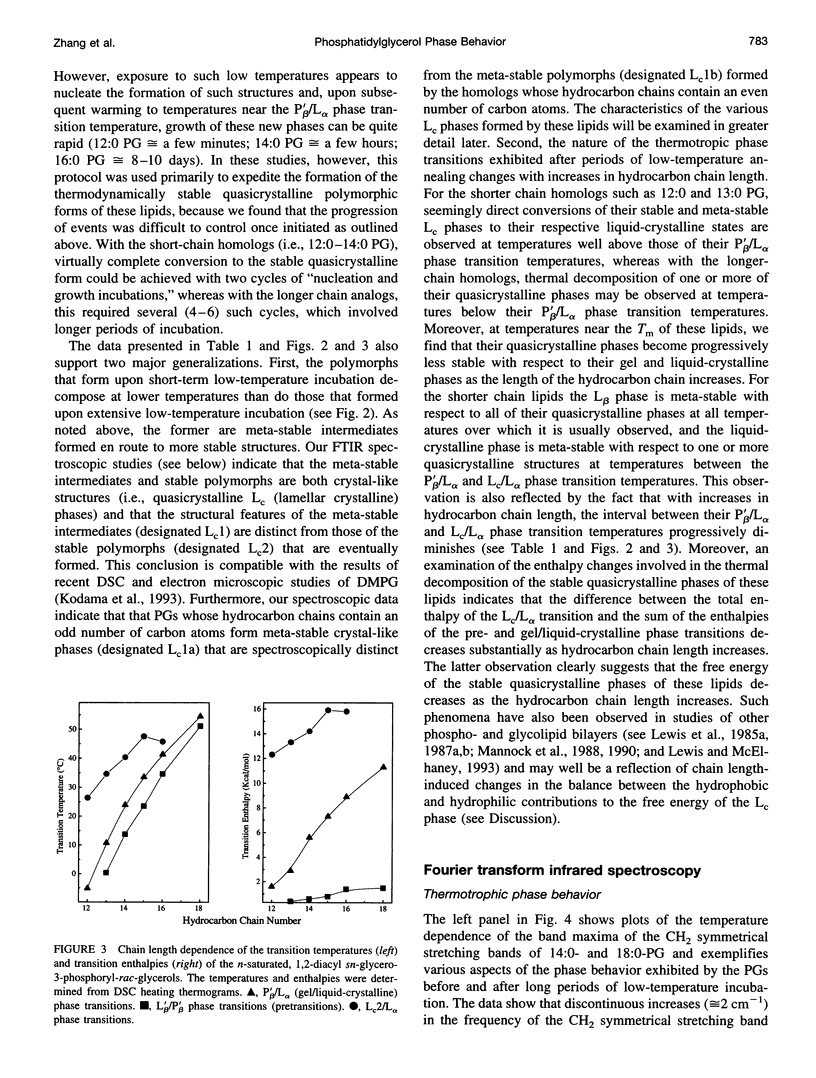
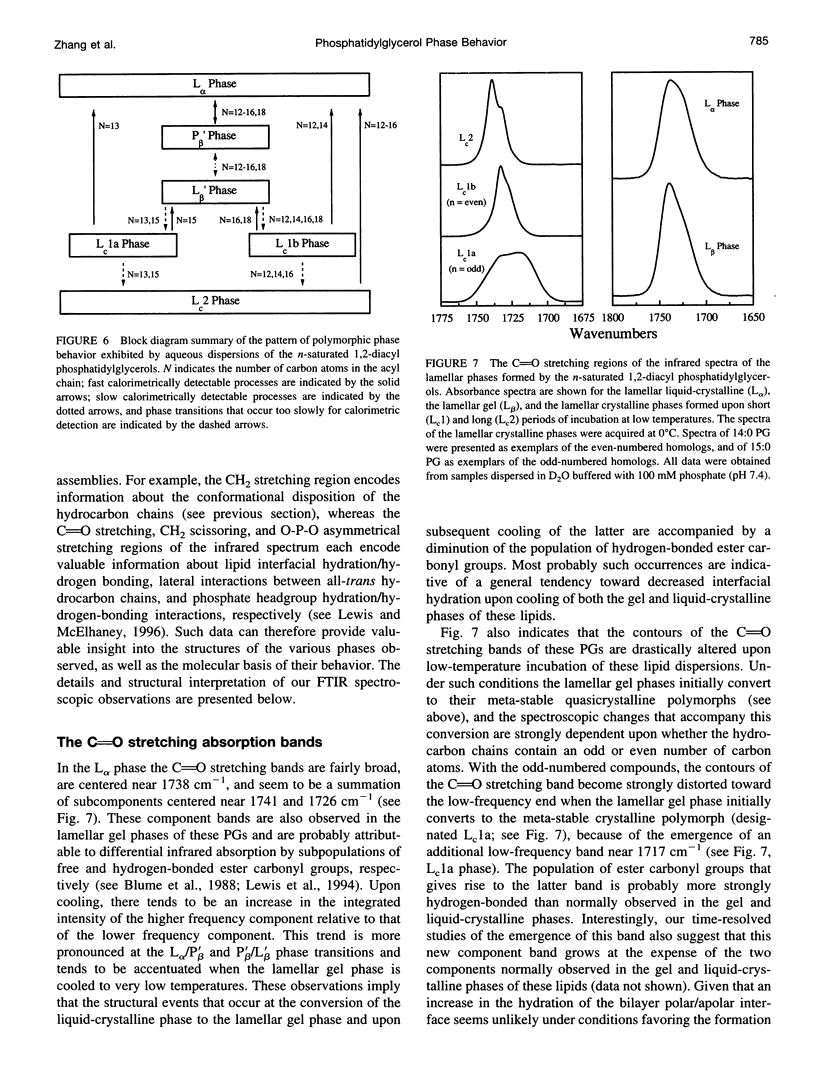
The polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylglycerols (PGs) was studied by differential scanning calorimetry and Fourier transform infrared and 31P-nuclear magnetic resonance spectroscopy. When dispersed in aqueous media under physiologically relevant conditions, these compounds exhibit two thermotropic phase transitions that are structurally equivalent to the well-characterized pretransitons and gel/liquid-crystalline phase transitions exhibited by bilayers of the corresponding 1,2-diacyl phosphatidylcholines. Furthermore, when incubated at low temperatures, their gel phases spontaneously transform into one or more solid-like phases that appear to be highly ordered, quasicrystalline bilayers that are probably partially dehydrated. The quasicrystalline structures, which form upon short-term, low-temperature annealing of these lipids, are meta-stable with respect to more stable structures, to which they eventually transform upon prolonged low-temperature incubation. The rates of formation of the quasicrystalline phases of the PGs generally tend to decrease as hydrocarbon chain length increases, and PGs whose hydrocarbon chains contain an odd number of carbon atoms tend to be slower than those of neighboring even-numbered homologs. The calorimetric data also indicate that the quasicrystalline phases of these compounds become progressively less stable relative to both their gel and liquid-crystalline phases as the length of the hydrocarbon chain increases and that they decompose either to the liquid-crystalline phase (short- and medium-chain compounds) or to the normal gel phase (long-chain compounds) upon heating. The spectroscopic data indicate that although there is odd-even alternation in the structures of the quasicrystalline phases formed upon short-term low-temperature incubation of these compounds, the structural features of the stable quasicrystalline phases eventually formed are all similar. Furthermore, the degree of hydration and the nature of hydrogen bonding interactions in the headgroup and interfacial regions of these PG bilayers differ significantly from that observed in all other phospholipid bilayers studied so far. We suggest that many of the properties of PG bilayers can be rationalized by postulating that the glycerol moiety of the polar headgroup is directly involved in shielding the negative charges at the surface of the bilayer by means of hydration-like hydrogen bonding interactions with the phosphate moiety.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E., McIntosh T. J. Structure of the crystalline bilayer in the subgel phase of dipalmitoylphosphatidylglycerol. Biochemistry. 1986 Jan 28;25(2):299–305. doi: 10.1021/bi00350a003. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Effect of lipid structural modifications on their intermolecular hydrogen bonding interactions and membrane functions. Biochem Cell Biol. 1986 Jan;64(1):50–57. doi: 10.1139/o86-008. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can J Biochem. 1980 Oct;58(10):755–770. doi: 10.1139/o80-107. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Moscarello M. A. Comparison of metastable phase behavior of the complexes of dipalmitoyl phosphatidylglycerol with Mg+2 and myelin basic protein. Can J Biochem Cell Biol. 1984 Jan;62(1):11–18. doi: 10.1139/o84-003. [DOI] [PubMed] [Google Scholar]
- Cevc G. How membrane chain melting properties are regulated by the polar surface of the lipid bilayer. Biochemistry. 1987 Oct 6;26(20):6305–6310. doi: 10.1021/bi00394a002. [DOI] [PubMed] [Google Scholar]
- Christiansson A., Eriksson L. E., Westman J., Demel R., Wieslander A. Involvement of surface potential in regulation of polar membrane lipids in Acholeplasma laidlawii. J Biol Chem. 1985 Apr 10;260(7):3984–3990. [PubMed] [Google Scholar]
- Church S. E., Griffiths D. J., Lewis R. N., McElhaney R. N., Wickman H. H. X-ray structure study of thermotropic phases in isoacylphosphatidylcholine multibilayers. Biophys J. 1986 Mar;49(3):597–605. doi: 10.1016/S0006-3495(86)83687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz T., Christiansson A., Wieslander A. Membrane potential, lipid regulation and adenylate energy charge in acyl chain modified Acholeplasma laidlawii. Biochim Biophys Acta. 1987 Apr 23;898(3):299–307. doi: 10.1016/0005-2736(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Clementz T., Christiansson A., Wieslander A. Transmembrane electrical potential affects the lipid composition of Acholeplasma laidlawii. Biochemistry. 1986 Feb 25;25(4):823–830. doi: 10.1021/bi00352a014. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Gabel B., Epand R. F., Sen A., Hui S. W., Muga A., Surewicz W. K. Formation of a new stable phase of phosphatidylglycerols. Biophys J. 1992 Aug;63(2):327–332. doi: 10.1016/S0006-3495(92)81618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay E. J., Barton P. G. Phase behavior of synthetic phosphatidylglycerols and binary mixtures with phosphatidylcholines in the presence and absence of calcium ions. Biochemistry. 1978 Jun 13;17(12):2400–2405. doi: 10.1021/bi00605a023. [DOI] [PubMed] [Google Scholar]
- George R., Lewis R. N., Mahajan S., McElhaney R. N. Studies on the purified, lipid-reconstituted (Na+ + Mg2+)-ATPase from Acholeplasma laidlawii B membranes. Dependence of enzyme activity on lipid headgroup and hydrocarbon chain structure. J Biol Chem. 1989 Jul 15;264(20):11598–11604. [PubMed] [Google Scholar]
- Heimburg T., Biltonen R. L. Thermotropic behavior of dimyristoylphosphatidylglycerol and its interaction with cytochrome c. Biochemistry. 1994 Aug 16;33(32):9477–9488. doi: 10.1021/bi00198a013. [DOI] [PubMed] [Google Scholar]
- Huunan-Seppälä A. Cation permeability induced by spermine and Polybrene in rat liver mitochondria. J Bioenerg. 1971 Aug;2(3):197–207. doi: 10.1007/BF01648914. [DOI] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M., Miyata T., Yokoyama T. Crystalline cylindrical structures of Na(+)-bound dimyristoylphosphatidylglycerol as revealed by microcalorimetry and electron microscopy. Biochim Biophys Acta. 1993 Jun 12;1168(2):243–248. [PubMed] [Google Scholar]
- Lewis R. N., Mak N., McElhaney R. N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry. 1987 Sep 22;26(19):6118–6126. doi: 10.1021/bi00393a026. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mantsch H. H., McElhaney R. N. Thermotropic phase behavior of phosphatidylcholines with omega-tertiary-butyl fatty acyl chains. Biophys J. 1989 Jul;56(1):183–193. doi: 10.1016/S0006-3495(89)82663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines. Biophys J. 1993 Apr;64(4):1081–1096. doi: 10.1016/S0006-3495(93)81474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N., Pohle W., Mantsch H. H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: a reevaluation. Biophys J. 1994 Dec;67(6):2367–2375. doi: 10.1016/S0006-3495(94)80723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Structures of the subgel phases of n-saturated diacyl phosphatidylcholine bilayers: FTIR spectroscopic studies of 13C = O and 2H labeled lipids. Biophys J. 1992 Jan;61(1):63–77. doi: 10.1016/S0006-3495(92)81816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Subgel phases of n-saturated diacylphosphatidylcholines: a Fourier-transform infrared spectroscopic study. Biochemistry. 1990 Aug 28;29(34):7946–7953. doi: 10.1021/bi00486a024. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing omega-cyclohexyl fatty acids. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1985 Aug 27;24(18):4903–4911. doi: 10.1021/bi00339a027. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing cis-monounsaturated acyl chain homologues of oleic acid: differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1988 Feb 9;27(3):880–887. doi: 10.1021/bi00403a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing dl-methyl anteisobranched fatty acids. 1. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1987 Jun 30;26(13):4036–4044. doi: 10.1021/bi00387a044. [DOI] [PubMed] [Google Scholar]
- Lipowsky R. The morphology of lipid membranes. Curr Opin Struct Biol. 1995 Aug;5(4):531–540. doi: 10.1016/0959-440x(95)80040-9. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Leisen J., Marassi F. M. Response of phosphatidylcholine in the gel and liquid-crystalline states to membrane surface charges. Biochemistry. 1991 Apr 9;30(14):3558–3566. doi: 10.1021/bi00228a029. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 1. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7790–7799. doi: 10.1021/bi00486a003. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., Sen A., McElhaney R. N. The physical properties of glycosyldiacylglycerols. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(beta-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1988 Sep 6;27(18):6852–6859. doi: 10.1021/bi00418a030. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. An infrared spectroscopic study of the thermotropic phase behavior of phosphatidylcholines containing omega-cyclohexyl fatty acyl chains. Biochim Biophys Acta. 1989 Mar 27;980(1):42–49. doi: 10.1016/0005-2736(89)90198-3. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing dl-methyl anteisobranched fatty acids. 2. An infrared spectroscopy study. Biochemistry. 1987 Jun 30;26(13):4045–4049. doi: 10.1021/bi00387a045. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 2. Infrared and 31P NMR spectroscopic studies. Biochemistry. 1985 May 7;24(10):2440–2446. doi: 10.1021/bi00331a008. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G., Ciani S. M. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1268–1275. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. J., Wyrwa M., Reboulleau C. P., Mendelsohn R. Quantitative IR studies of acyl chain conformational order in fatty acid homogeneous membranes of live cells of Acholeplasma laidlawii B. Biochemistry. 1993 Jun 22;32(24):6281–6287. doi: 10.1021/bi00075a023. [DOI] [PubMed] [Google Scholar]
- Pascher I., Sundell S., Harlos K., Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta. 1987 Jan 9;896(1):77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro T. J., Watts A. Lipid specificity in the interaction of cytochrome c with anionic phospholipid bilayers revealed by solid-state 31P NMR. Biochemistry. 1994 Mar 8;33(9):2451–2458. doi: 10.1021/bi00175a013. [DOI] [PubMed] [Google Scholar]
- Schäfer G., Rowohl-Quisthoudt G. Influence of electrostatic surface potential on mitochondrial ADP-phosphorylation. FEBS Lett. 1975 Nov 1;59(1):48–51. doi: 10.1016/0014-5793(75)80338-3. [DOI] [PubMed] [Google Scholar]
- Schäfer G., Rowohl-Quisthoudt G. Influence of surface potentials on the mitochondrial H+ pump and on lipid-phase transitions. J Bioenerg. 1976 Apr;8(2):73–81. doi: 10.1007/BF01558629. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Sen A., Hui S. W., Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 2. X-ray diffraction studies of a homologous series of 1,2-Di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7799–7804. doi: 10.1021/bi00486a004. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Epand R. M. Phospholipid structure determines the effects of peptides on membranes. Differential scanning calorimetry studies with pentagastrin-related peptides. Biochim Biophys Acta. 1986 Apr 14;856(2):290–300. doi: 10.1016/0005-2736(86)90039-8. [DOI] [PubMed] [Google Scholar]
- Ter-Minassian-Saraga L., Okamura E., Umemura J., Takenaka T. Fourier transform infrared-attenuated total reflection spectroscopy of hydration of dimyristoylphosphatidylcholine multibilayers. Biochim Biophys Acta. 1988 Dec 22;946(2):417–423. doi: 10.1016/0005-2736(88)90417-8. [DOI] [PubMed] [Google Scholar]
- Theretz A., Ranck J. L., Tocanne J. F. Polymyxin B-induced phase separation and acyl chain interdigitation in phosphatidylcholine/phosphatidylglycerol mixtures. Biochim Biophys Acta. 1983 Aug 10;732(3):499–508. doi: 10.1016/0005-2736(83)90226-2. [DOI] [PubMed] [Google Scholar]
- Theuvenet A. P., Borst-Pauwels G. W. Kinetics of ion translocation across charged membranes mediated by a two-site transport mechanism. Effects of polyvalent cations upon rubidium uptake into yeast cells. Biochim Biophys Acta. 1976 Apr 5;426(4):745–756. doi: 10.1016/0005-2736(76)90139-5. [DOI] [PubMed] [Google Scholar]
- Theuvenet A. P., Borst-Pauwels G. W. The influence of surface charge on the kinetics of ion-translocation across biological membranes. J Theor Biol. 1976 Apr;57(2):313–329. doi: 10.1016/0022-5193(76)90004-7. [DOI] [PubMed] [Google Scholar]
- Watts A., Harlos K., Marsh D. Charge-induced tilt in ordered-phase phosphatidylglycerol bilayers evidence from X-ray diffraction. Biochim Biophys Acta. 1981 Jul 6;645(1):91–96. doi: 10.1016/0005-2736(81)90515-0. [DOI] [PubMed] [Google Scholar]
- Watts A., Harlos K., Maschke W., Marsh D. Control of the structure and fluidity of phosphatidylglycerol bilayers by pH titration. Biochim Biophys Acta. 1978 Jun 16;510(1):63–74. doi: 10.1016/0005-2736(78)90130-x. [DOI] [PubMed] [Google Scholar]
- Watts A., Marsh D. Saturation transfer ESR studies of molecular motion in phosphatidylglycerol bilayers in the gel phase: effects of pretransitions and pH titration. Biochim Biophys Acta. 1981 Apr 6;642(2):231–241. doi: 10.1016/0005-2736(81)90442-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., McIntosh T. J. A subtransition in a phospholipid with a net charge, dipalmitoylphosphatidylglycerol. Biochemistry. 1986 Jan 28;25(2):295–298. doi: 10.1021/bi00350a002. [DOI] [PubMed] [Google Scholar]
- Williamson P., Schlegel R. A. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol. 1994 Oct-Dec;11(4):199–216. doi: 10.3109/09687689409160430. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. 31P NMR relaxation studies of the activation of the coenzyme phosphate of glycogen phosphorylase. The role of motion of the bound phosphate. Biophys J. 1985 Dec;48(6):1019–1026. doi: 10.1016/S0006-3495(85)83864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Nałecz M. J. Surface change of biological membranes as a possible regulator of membrane-bound enzymes. Eur J Biochem. 1979 Feb 15;94(1):99–107. doi: 10.1111/j.1432-1033.1979.tb12876.x. [DOI] [PubMed] [Google Scholar]
- Xia W., Dowhan W. In vivo evidence for the involvement of anionic phospholipids in initiation of DNA replication in Escherichia coli. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):783–787. doi: 10.1073/pnas.92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Dowhan W. Phosphatidylinositol cannot substitute for phosphatidylglycerol in supporting cell growth of Escherichia coli. J Bacteriol. 1995 May;177(10):2926–2928. doi: 10.1128/jb.177.10.2926-2928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Stephenson F. A., Lin H. N., Huang C. H. Phase metastability and supercooled metastable state of diundecanoylphosphatidylethanolamine bilayers. Biochim Biophys Acta. 1988 Aug 4;943(1):63–75. doi: 10.1016/0005-2736(88)90347-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Fukumori Y., Yamanaka T. Catalytic properties of cytochrome c oxidase purified from Nitrosomonas europaea. J Biochem. 1988 Mar;103(3):499–503. doi: 10.1093/oxfordjournals.jbchem.a122299. [DOI] [PubMed] [Google Scholar]
- de Jongh H. H., Ritsema T., Killian J. A. Lipid specificity for membrane mediated partial unfolding of cytochrome c. FEBS Lett. 1995 Mar 6;360(3):255–260. doi: 10.1016/0014-5793(95)00115-p. [DOI] [PubMed] [Google Scholar]
- van der Goot F. G., Didat N., Pattus F., Dowhan W., Letellier L. Role of acidic lipids in the translocation and channel activity of colicins A and N in Escherichia coli cells. Eur J Biochem. 1993 Apr 1;213(1):217–221. doi: 10.1111/j.1432-1033.1993.tb17751.x. [DOI] [PubMed] [Google Scholar]


