Abstract
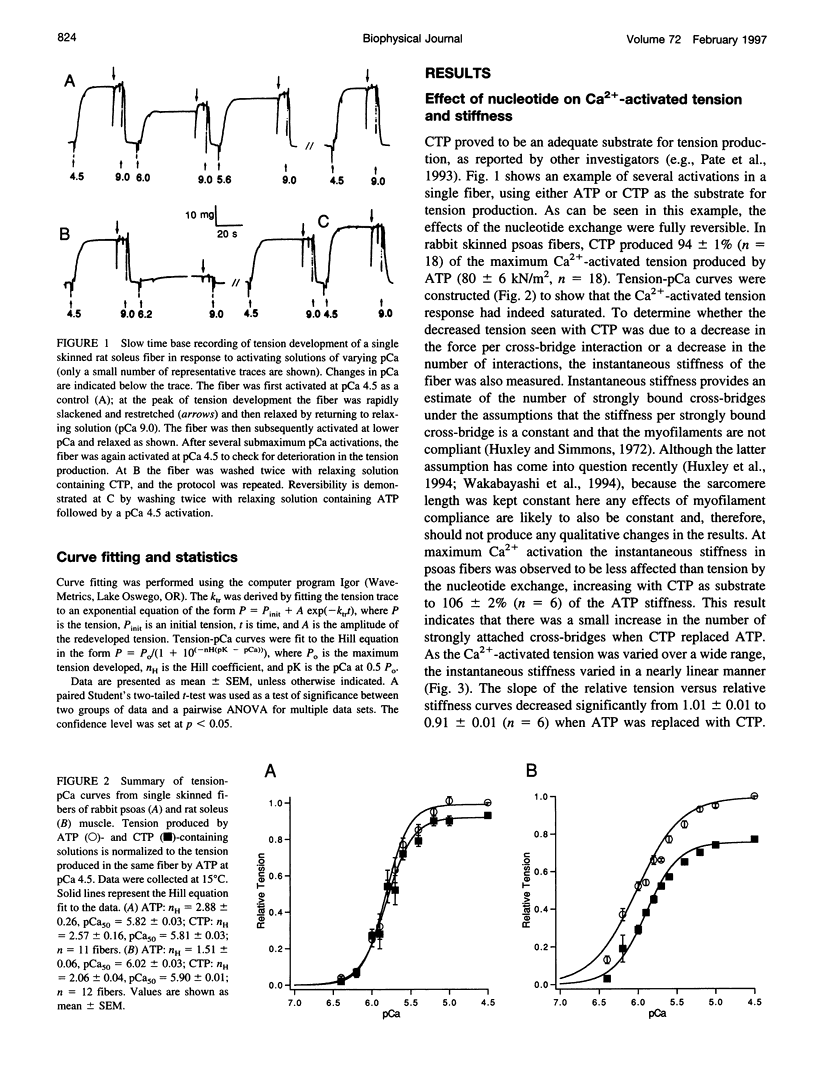
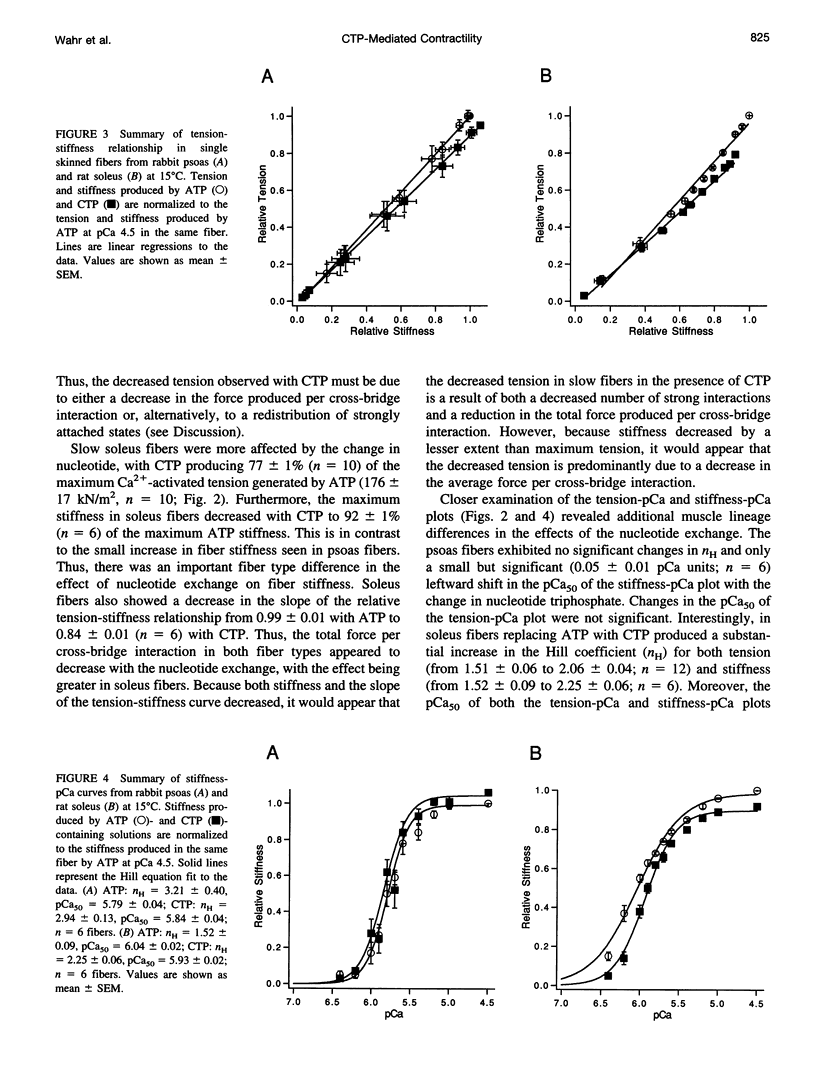
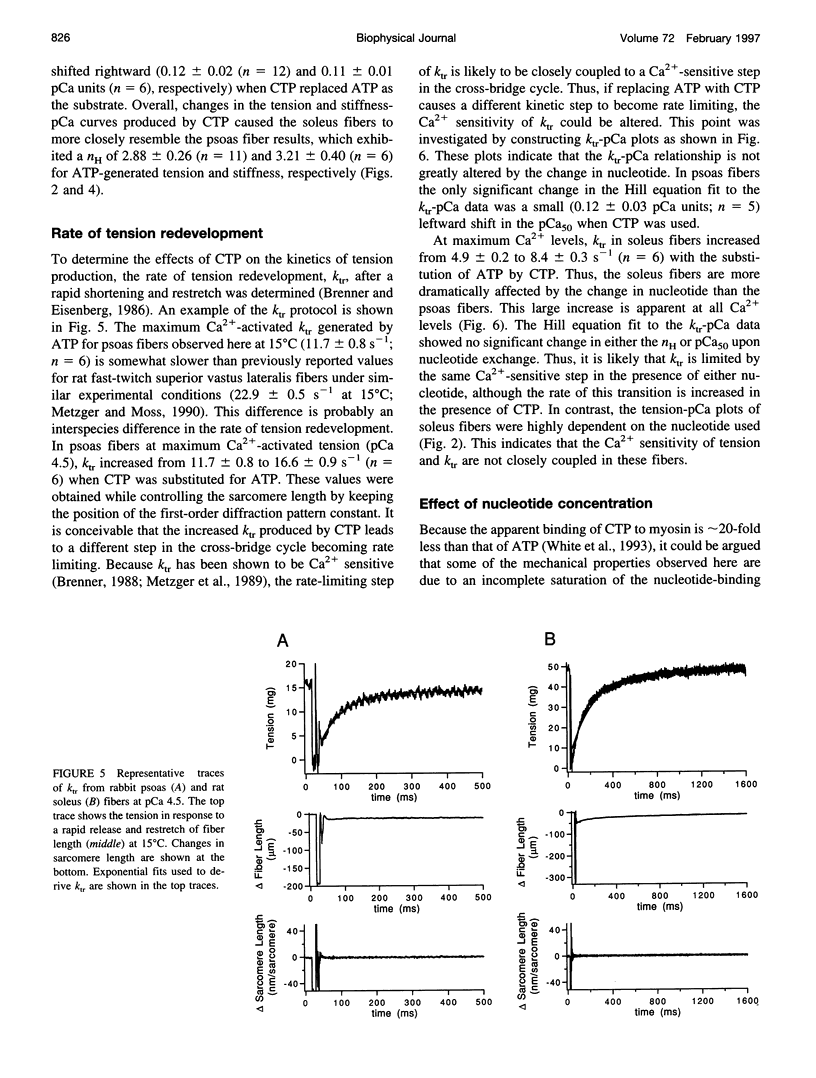
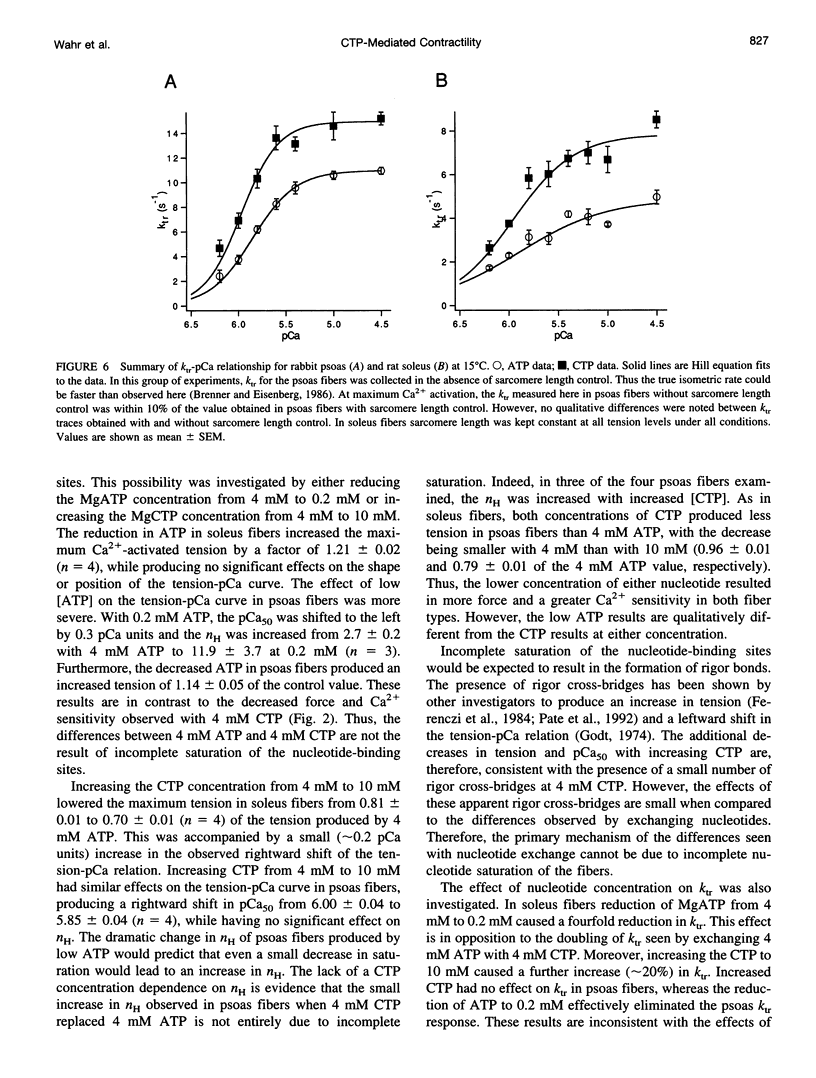
The relation between single skinned skeletal fiber contractile mechanics and the myosin mechanoenzyme was examined by perturbing the actomyosin interaction with the ATP analog CTP in fibers from both rabbit psoas and rat soleus. Tension, instantaneous stiffness, and the rate of tension redevelopment (ktr), under software-based sarcomere length control, were examined at 15°C for a range of Ca2+ concentrations in both fiber types. CTP produced 94% of the maximum ATP-generated tension in psoas fibers and 77% in soleus fibers. In psoas, CTP also increased stiffness to 106% of the ATP stiffness, whereas in soleus stiffness decreased to 92%. Thus, part of the greater difference between maximum ATP- and CTP-generated tension in soleus fibers appears to be due to a decrease in strongly bound cross-bridge number. Interestingly, although the nucleotide exchange produced substantial increases in the steepness (nH) of the tension- and stiffness-pCa relationships in soleus fibers, only minor changes were seen in psoas fibers. At maximum Ca2+ and nominal Pi levels, ktr in psoas fibers increased from 11.7 s-1 with ATP to 16.6 s-1 with CTP and in soleus fibers from 4.9 to 8.4 s-1. Increased Pi levels decreased the maximum Ca2+-activated tension in both fiber types and increased the ktr of psoas fibers, but the ktr of soleus fibers was not significantly altered. Thus, although the nucleotide exchange generally produced similar changes in the mechanics, there were significant muscle lineage differences in the tension- and stiffness-pCa relations and in the effects of Pi on ktr, such that differences in contractile mechanics were lessened in the presence of CTP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukatina A. E., Fuchs F., Brandt P. W. Thin filament activation by phalloidin in skinned cardiac muscle. J Mol Cell Cardiol. 1995 Jun;27(6):1311–1315. doi: 10.1016/s0022-2828(05)82393-4. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Selden L. A., Gershman L. C. Mechanism of action of phalloidin on the polymerization of muscle actin. Biochemistry. 1981 Feb 17;20(4):708–712. doi: 10.1021/bi00507a006. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Extra calcium on shortening in barnacle muscle. Is the decrease in calcium binding related to decreased cross-bridge attachment, force, or length? J Gen Physiol. 1987 Sep;90(3):321–340. doi: 10.1085/jgp.90.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros F., Buckingham M. Polymorphism of contractile proteins. Biopolymers. 1987;26 (Suppl):S177–S192. doi: 10.1002/bip.360260016. [DOI] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. E. Alternate model for the cooperative equilibrium binding of myosin subfragment-1-nucleotide complex to actin-troponin-tropomyosin. Proc Natl Acad Sci U S A. 1983 Jan;80(1):60–64. doi: 10.1073/pnas.80.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Millar N. C. Caged compounds and striated muscle contraction. Annu Rev Physiol. 1990;52:875–896. doi: 10.1146/annurev.ph.52.030190.004303. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994 Dec;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Kinetics of the "on-off" change in regulatory state of the muscle thin filament. Arch Biochem Biophys. 1993 Aug 15;305(1):193–196. doi: 10.1006/abbi.1993.1410. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Oster G. F., Lamb J., Stossel T. P., Hartwig J. H. Effect of ATP on actin filament stiffness. Nature. 1990 Sep 6;347(6288):95–99. doi: 10.1038/347095a0. [DOI] [PubMed] [Google Scholar]
- Kawai M., Zhao Y. Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophys J. 1993 Aug;65(2):638–651. doi: 10.1016/S0006-3495(93)81109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., GOUVEA M. A., GERGERLY J. Studies on actin. I. The interaction of C14-labeled adenine nucleotides with actin. J Biol Chem. 1960 Jun;235:1700–1703. [PubMed] [Google Scholar]
- McKillop D. F., Geeves M. A. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993 Aug;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Greaser M. L., Moss R. L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989 May;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science. 1990 Mar 2;247(4946):1088–1090. doi: 10.1126/science.2309121. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Myosin light chain 2 modulates calcium-sensitive cross-bridge transitions in vertebrate skeletal muscle. Biophys J. 1992 Aug;63(2):460–468. doi: 10.1016/S0006-3495(92)81614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am J Physiol. 1992 May;262(5 Pt 1):C1239–C1245. doi: 10.1152/ajpcell.1992.262.5.C1239. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Zaner K. S., Schick K. L., Gershman L. C., Selden L. A., Kinosian H. J., Travis J. L., Estes J. E. Nucleotide exchange and rheometric studies with F-actin prepared from ATP- or ADP-monomeric actin. Biophys J. 1993 May;64(5):1559–1566. doi: 10.1016/S0006-3495(93)81525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N., Sugi H. In vitro ATP-dependent F-actin sliding on myosin is not influenced by substitution or removal of bound nucleotide. Biochim Biophys Acta. 1994 May 18;1185(3):346–349. doi: 10.1016/0005-2728(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 1989 May;414(1):73–81. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- Pate E., Franks-Skiba K., White H., Cooke R. The use of differing nucleotides to investigate cross-bridge kinetics. J Biol Chem. 1993 May 15;268(14):10046–10053. [PubMed] [Google Scholar]
- Pate E., Lin M., Franks-Skiba K., Cooke R. Contraction of glycerinated rabbit slow-twitch muscle fibers as a function of MgATP concentration. Am J Physiol. 1992 Apr;262(4 Pt 1):C1039–C1046. doi: 10.1152/ajpcell.1992.262.4.C1039. [DOI] [PubMed] [Google Scholar]
- Pate E., Nakamaye K. L., Franks-Skiba K., Yount R. G., Cooke R. Mechanics of glycerinated muscle fibers using nonnucleoside triphosphate substrates. Biophys J. 1991 Mar;59(3):598–605. doi: 10.1016/S0006-3495(91)82275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M., Morris C., Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am J Physiol. 1995 Dec;269(6 Pt 1):C1532–C1539. doi: 10.1152/ajpcell.1995.269.6.C1532. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
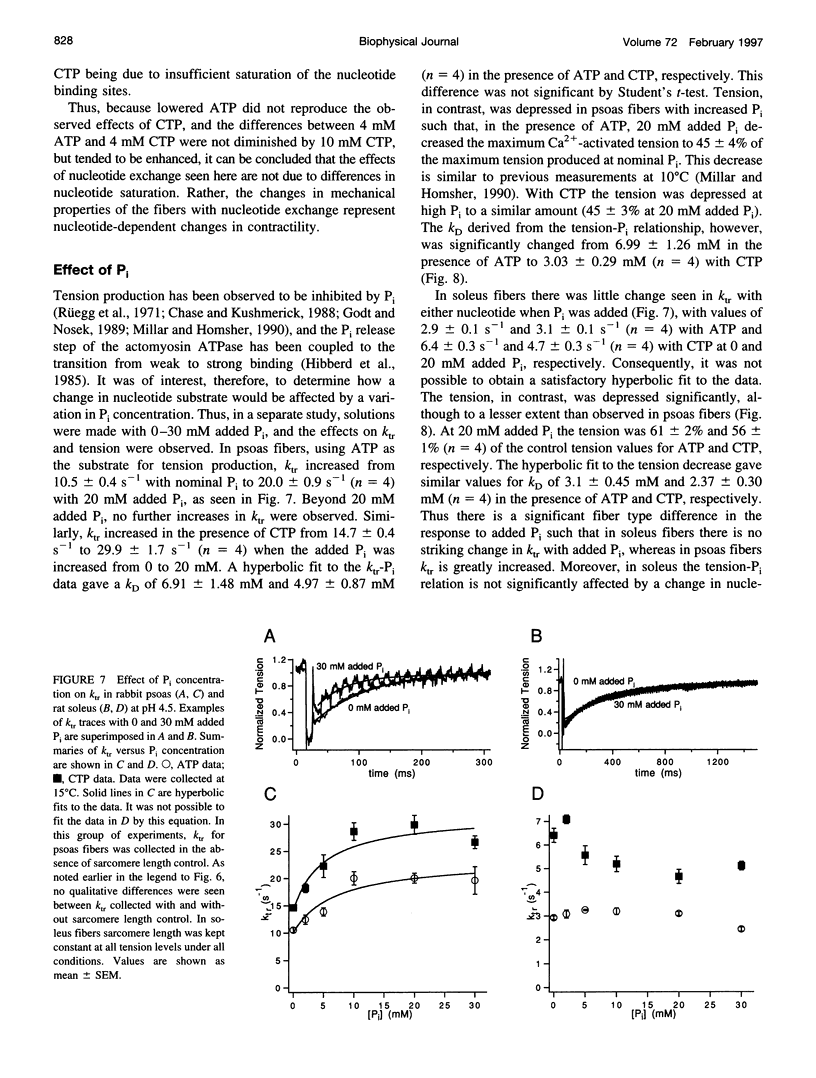
- Rüegg J. C., Schädler M., Steiger G. J., Müller G. Effects of inorganic phosphate on the contractile mechanism. Pflugers Arch. 1971;325(4):359–364. doi: 10.1007/BF00592176. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Belknap B., Jiang W. Kinetics of binding and hydrolysis of a series of nucleoside triphosphates by actomyosin-S1. Relationship between solution rate constants and properties of muscle fibers. J Biol Chem. 1993 May 15;268(14):10039–10045. [PubMed] [Google Scholar]



