Abstract
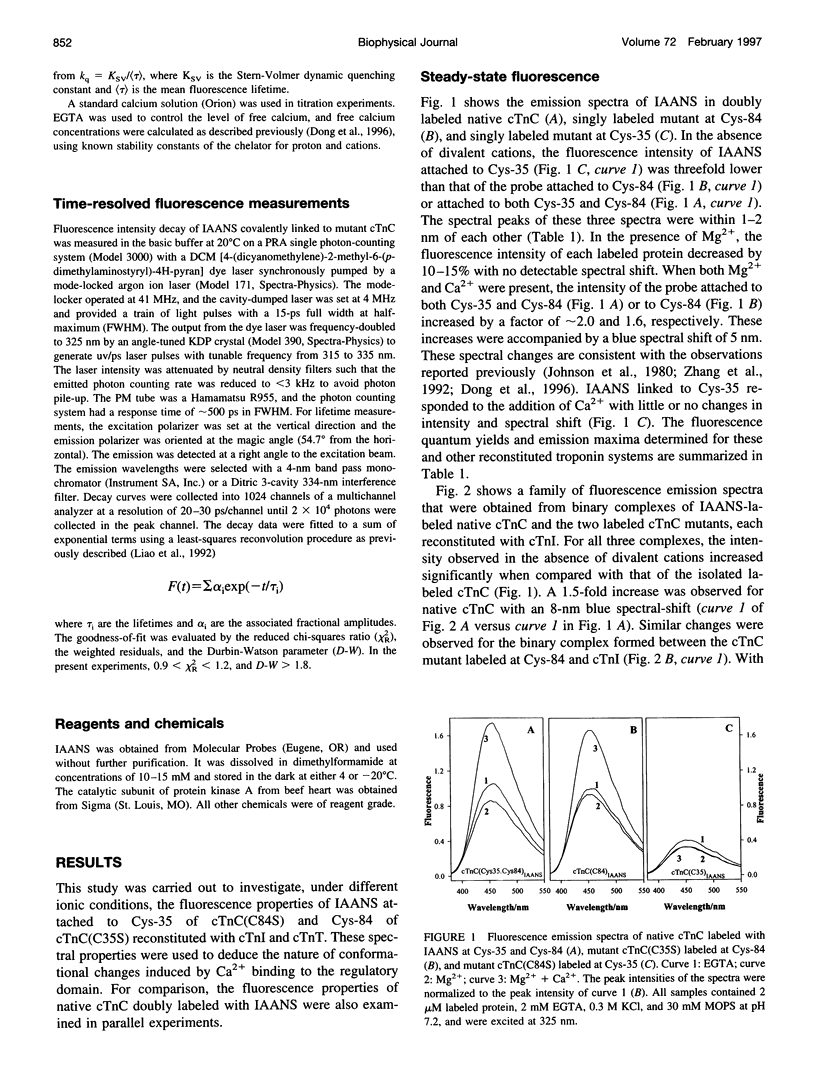
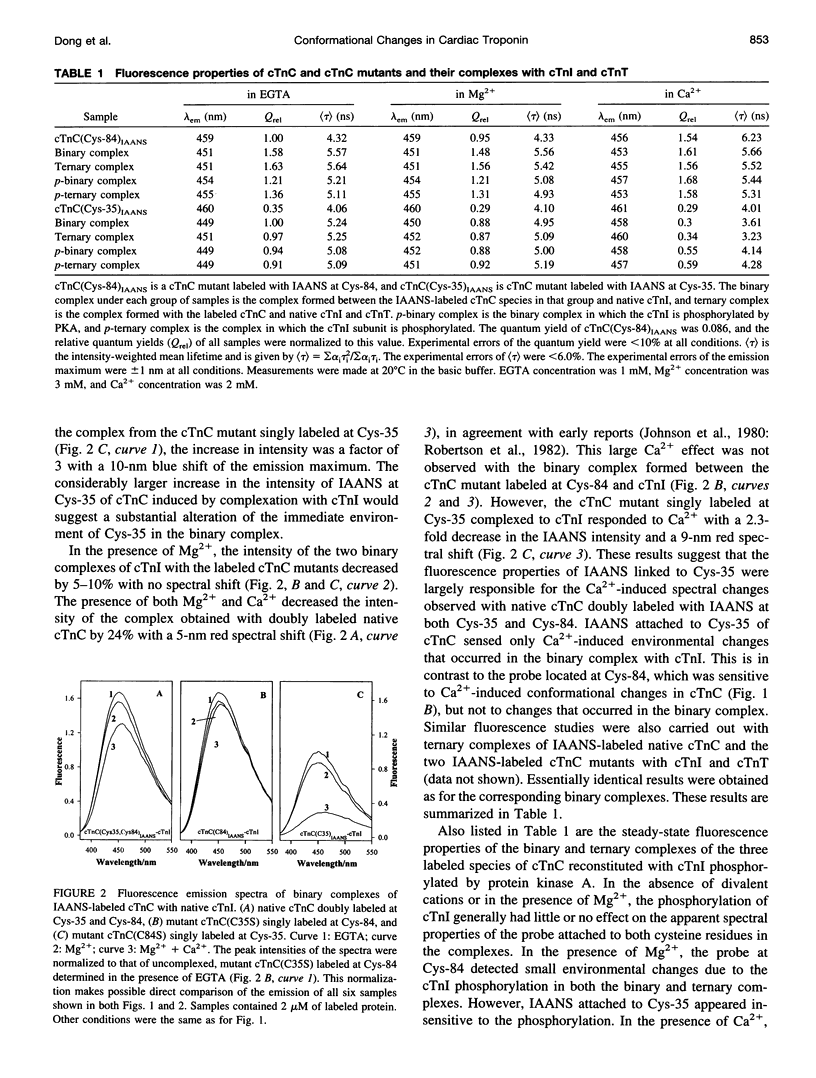
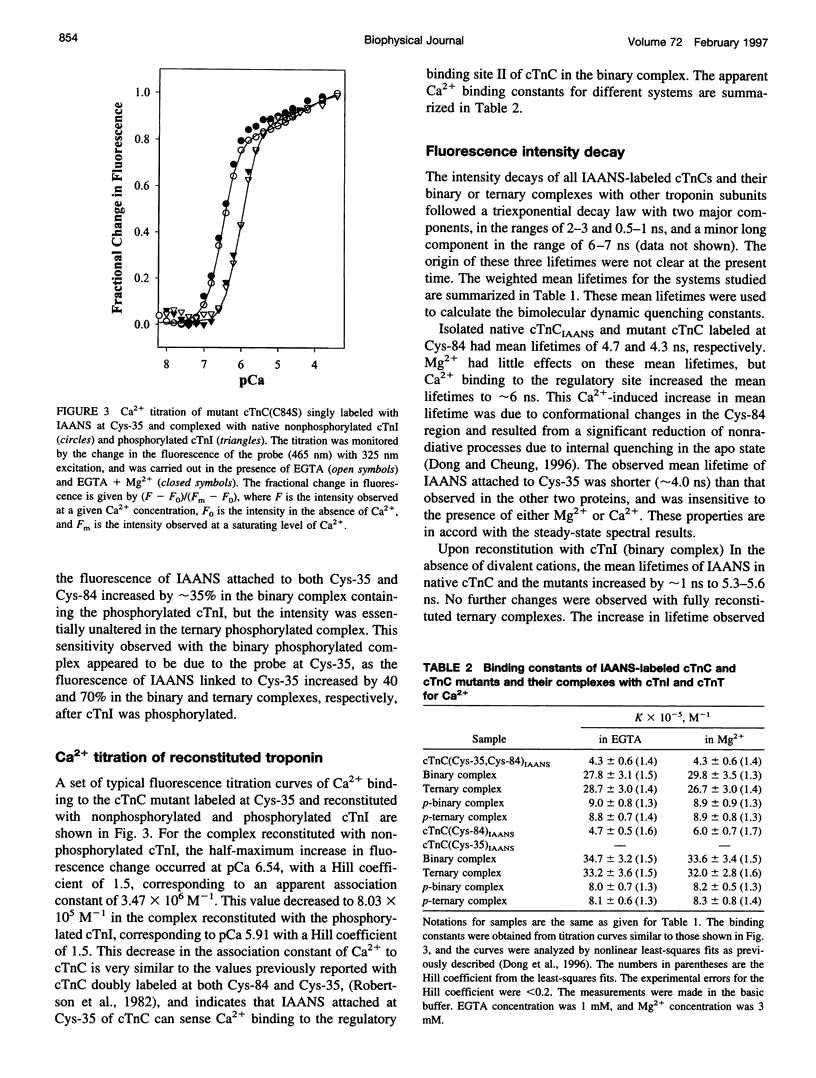
Two monocysteine mutants of cardiac muscle troponin C, cTnC(C35S) and cTnC(C84S), were genetically generated and labeled with the fluorescent probe 2-[4′-(iodoacetamido)anilino]naphthalene-6-sulfonic acid (IAANS) at Cys-84 and Cys-35, respectively. Cys-84 is located on helix D in the regulatory N-domain, and Cys-35 is at the -y position of the inactive 12-residue loop of site I. These labeled mutants were studied by a variety of steady-state and time-resolved fluorescence methods. In the absence of divalent cation, the fluorescence of the attached IAANS indicated an exposed environment at Cys-35 and a relatively less-exposed environment at Cys-84. The binding of Ca2+ to the single regulatory site elicited a large enhancement of the emission of IAANS attached to Cys-84, but only marginal fluorescence changes of the probe at Cys-35. Upon reconstitution of the labeled cTnC mutants with troponin I and troponin T to form the three-subunit troponin, the fluorescence of IAANS-Cys-84 in apo-troponin was spectrally similar to that observed with the Ca2+-loaded uncomplexed cTnC mutant. Only very moderate changes in the fluorescence of IAANS-Cys-84 were observed when the regulatory site in reconstituted troponin was saturated. The exposed Cys-35 environment of the uncomplexed cTnC mutant became considerably less exposed and less polar when the mutant was incorporated into apo-troponin. In contrast to the Cys-84 site, saturation of the regulatory site II by Ca2+ in reconstituted troponin resulted in a reversal of the environment of the Cys-35 site toward a more exposed and more polar environment. These results indicated involvement of the inactive loop I in the Ca2+ trigger mechanism in cardiac muscle. The fluorescence of IAANS at both Cys-84 and Cys-35 was sensitive to phosphorylation of cTnI in reconstituted troponin, and the sensitivity was observed with both apo-troponin and Ca2+-loaded troponin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Wang C. K., Malik N. A. Interactions of troponin subunits: free energy of binary and ternary complexes. Biochemistry. 1987 Sep 8;26(18):5904–5907. doi: 10.1021/bi00392a049. [DOI] [PubMed] [Google Scholar]
- Dong W. J., Cheung H. C. Calcium-induced conformational change in cardiac troponin C studied by fluorescence probes attached to Cys-84. Biochim Biophys Acta. 1996 Jul 18;1295(2):139–146. doi: 10.1016/0167-4838(96)00028-3. [DOI] [PubMed] [Google Scholar]
- Dong W., Rosenfeld S. S., Wang C. K., Gordon A. M., Cheung H. C. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. J Biol Chem. 1996 Jan 12;271(2):688–694. doi: 10.1074/jbc.271.2.688. [DOI] [PubMed] [Google Scholar]
- Gulati J., Babu A., Su H. Functional delineation of the Ca(2+)-deficient EF-hand in cardiac muscle, with genetically engineered cardiac-skeletal chimeric troponin C. J Biol Chem. 1992 Dec 15;267(35):25073–25077. [PubMed] [Google Scholar]
- Gulati J., Rao V. G. The cardiac Ca(2+)-deficient EF-hand governs the phenotype of the cardiac-skeletal TnC-chimera in solution by Sr(2+)-induced tryptophan fluorescence emission. Biochemistry. 1994 Aug 9;33(31):9052–9056. doi: 10.1021/bi00197a004. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. J Mol Biol. 1988 Oct 5;203(3):761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J., James M. N. A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J Biol Chem. 1986 Feb 25;261(6):2638–2644. [PubMed] [Google Scholar]
- Johnson J. D., Collins J. H., Robertson S. P., Potter J. D. A fluorescent probe study of Ca2+ binding to the Ca2+-specific sites of cardiac troponin and troponin C. J Biol Chem. 1980 Oct 25;255(20):9635–9640. [PubMed] [Google Scholar]
- Liao R., Wang C. K., Cheung H. C. Coupling of calcium to the interaction of troponin I with troponin C from cardiac muscle. Biochemistry. 1994 Oct 25;33(42):12729–12734. doi: 10.1021/bi00208a026. [DOI] [PubMed] [Google Scholar]
- Liao R., Wang C. K., Cheung H. C. Time-resolved tryptophan emission study of cardiac troponin I. Biophys J. 1992 Oct;63(4):986–995. doi: 10.1016/S0006-3495(92)81685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M. Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil. 1988 Oct;9(5):428–445. doi: 10.1007/BF01774069. [DOI] [PubMed] [Google Scholar]
- Ovaska M., Taskinen J. A model for human cardiac troponin C and for modulation of its Ca2+ affinity by drugs. Proteins. 1991;11(2):79–94. doi: 10.1002/prot.340110202. [DOI] [PubMed] [Google Scholar]
- Potter J. D. Preparation of troponin and its subunits. Methods Enzymol. 1982;85(Pt B):241–263. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Holroyde M. J., Kranias E. G., Potter J. D., Solaro R. J. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982 Jan 10;257(1):260–263. [PubMed] [Google Scholar]
- Satyshur K. A., Rao S. T., Pyzalska D., Drendel W., Greaser M., Sundaralingam M. Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-A resolution. J Biol Chem. 1988 Feb 5;263(4):1628–1647. [PubMed] [Google Scholar]
- el-Saleh S. C., Solaro R. J. Calmidazolium, a calmodulin antagonist, stimulates calcium-troponin C and calcium-calmodulin-dependent activation of striated muscle myofilaments. J Biol Chem. 1987 Dec 15;262(35):17240–17246. [PubMed] [Google Scholar]
- van Eerd J. P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976 Mar 9;15(5):1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]


