Abstract
1. Hepatocytes isolated from starved rats and incubated without other substrates oxidized ethanol at a rate of 0.8–0.9μmol/min per g wet wt. of cells. Addition of 10mm-lactate increased this rate 2-fold. 2. Quinolinate (5mm) or tryptophan (1mm) decreased the rate of gluconeogenesis with 10mm-lactate and 8mm-ethanol from 0.39 to 0.04–0.08μmol/min per g wet wt. of cells, but rates of ethanol oxidation were not decreased. From these results it appears that acceleration of ethanol oxidation by lactate is not dependent upon the stimulation of gluconeogenesis and the consequent increased demand for ATP. 3. As another test of the relationship between ethanol oxidation and gluconeogenesis, the initial lactate concentration was varied from 0.5mm to 10mm and pyruvate was added to give an initial [lactate]/[pyruvate] ratio of 10. This substrate combination gave a large stimulation of ethanol oxidation (from 0.8 to 2.6μmol/min per g wet wt. of cells) at low lactate concentrations (0.5–2.0mm), but rates remained nearly constant (2.6–3.0μmol/min per g wet wt. of cells) at higher lactate concentrations (2.0–10mm). 4. In contrast, owing to the presence of ethanol, the rate of glucose synthesis was only slightly increased (from 0.08 to 0.12μmol/min per g wet wt. of cells) between 0.5mm- and 2.0mm-lactate and continued to increase (from 0.12 to 0.65μmol/min per g wet wt. of cells) with lactate concentrations between 2 and 10mm. 5. In the presence of ethanol, O2 uptake increased with increasing substrate concentration over the entire range. 6. Changes in concentrations of glutamate and 2-oxoglutarate closely paralleled changes in the rate of ethanol oxidation. 7. In isolated hepatocytes, rates of ethanol oxidation are lower than those in vivo apparently because of depletion of malate–aspartate shuttle intermediates during cell preparation. Rates are returned to those observed in vivo by substrates that increase the intracellular concentration of shuttle metabolites.
Full text
PDF


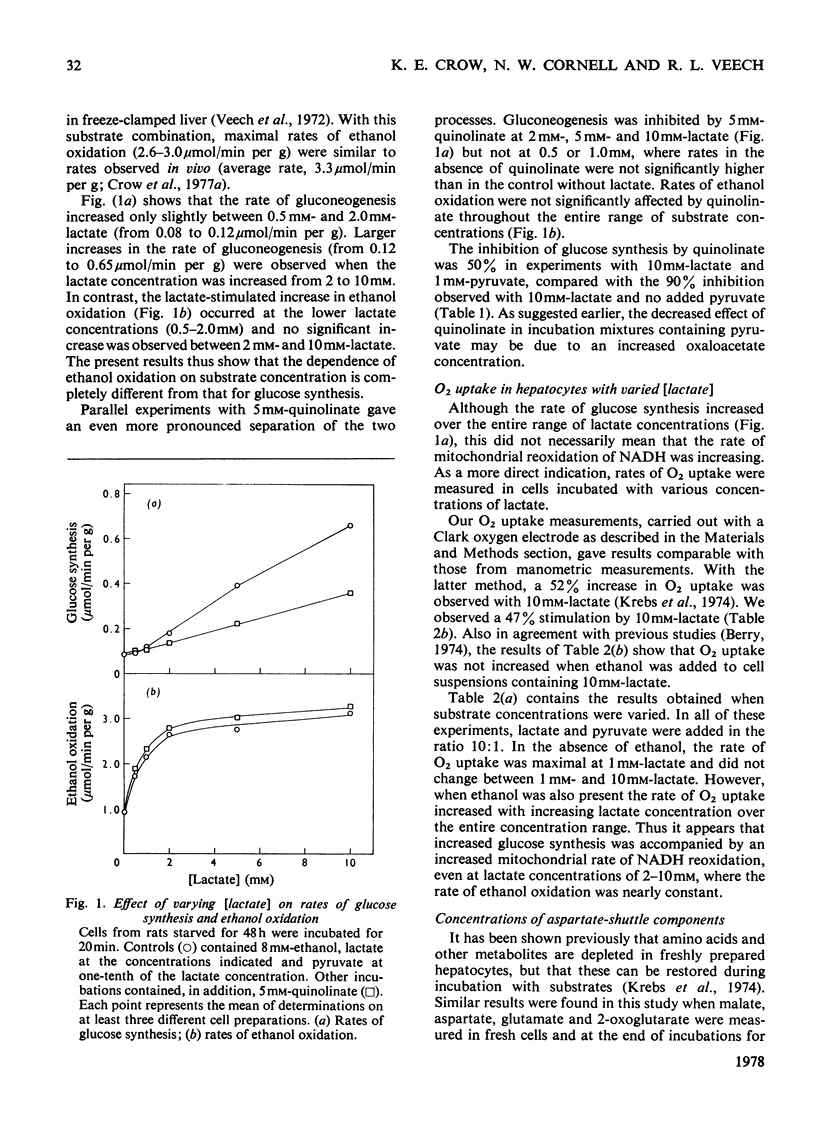
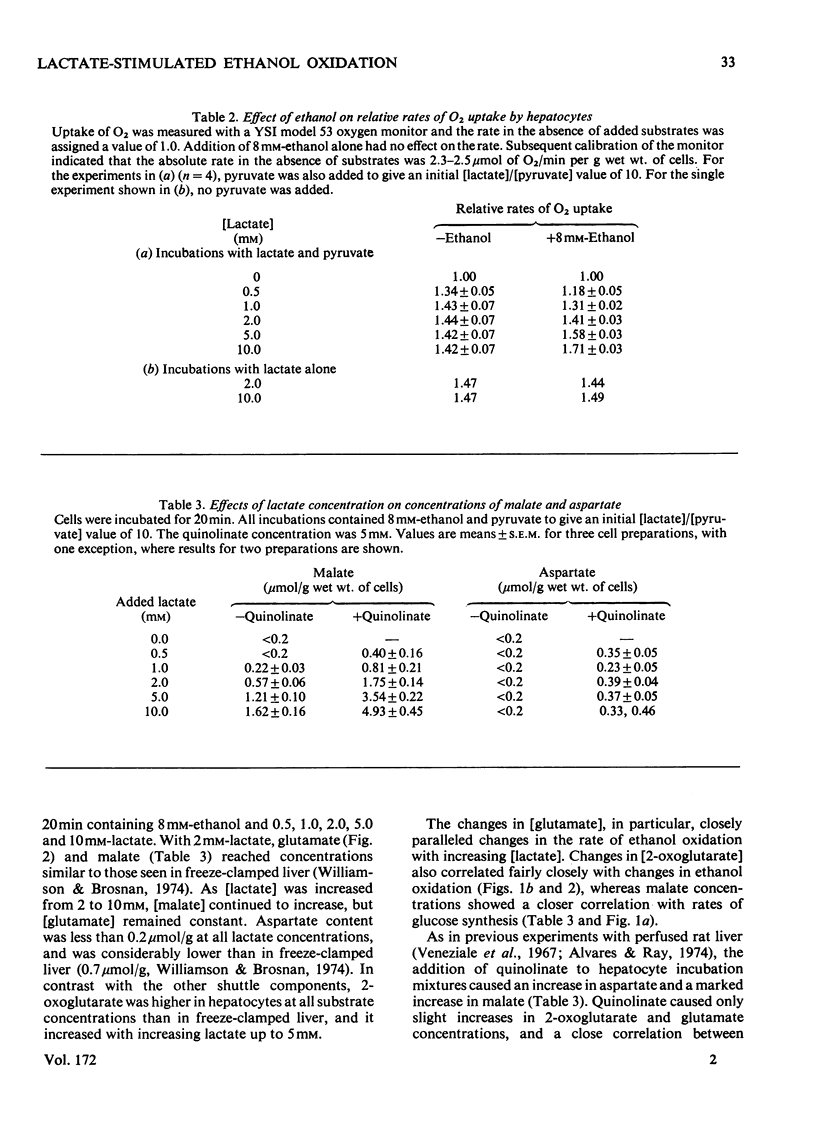
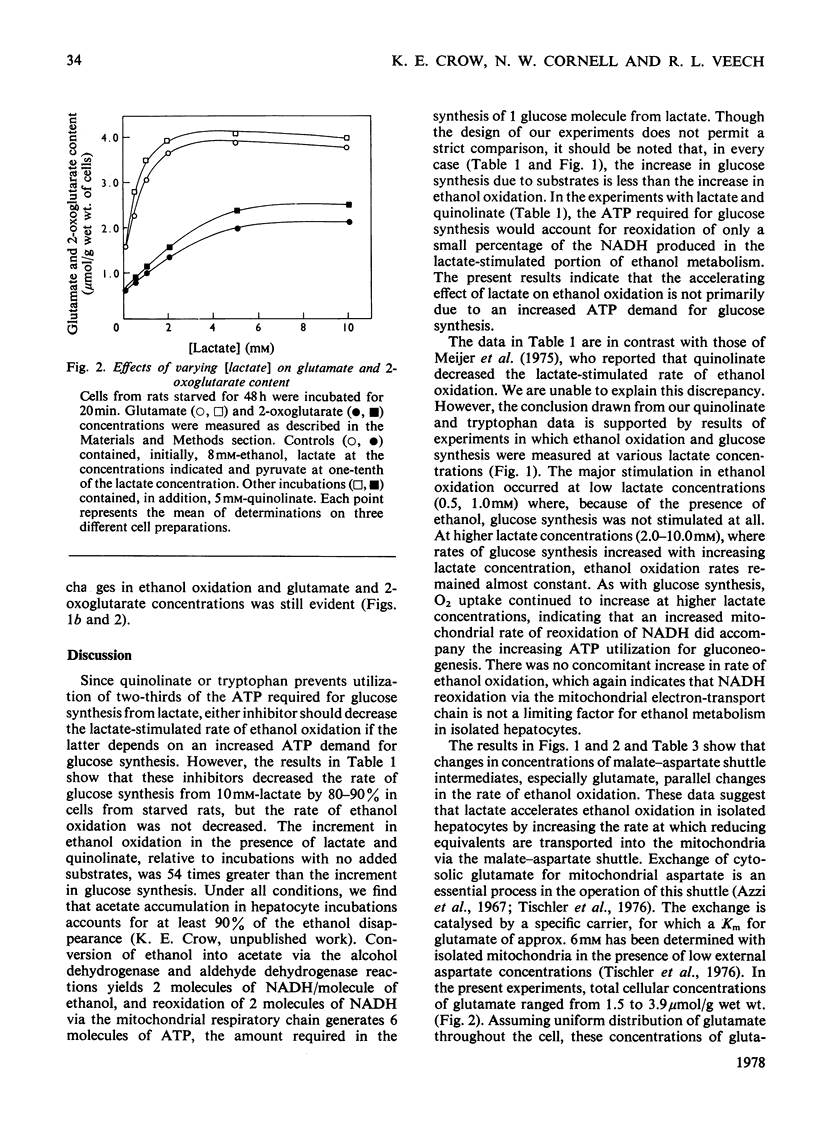


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares F. L., Ray P. D. Lack of inhibition by L-tryptophan or quinolinate of gluconeogenesis in diabetic rats. J Biol Chem. 1974 Apr 10;249(7):2058–2062. [PubMed] [Google Scholar]
- Azzi A., Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by glutamate and aspartate. Biochem Biophys Res Commun. 1967 Oct 11;29(1):148–152. doi: 10.1016/0006-291x(67)90556-6. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar R. B., Knappenberger M., Little B. Xanthine oxidase for calibration of the oxygen electrode apparatus. Anal Biochem. 1970 Jul;36(1):101–104. doi: 10.1016/0003-2697(70)90336-2. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Dalziel K. The specificities and configurations of ternary complexes of yeast and liver alcohol dehydrogenases. Biochem J. 1967 Jul;104(1):165–172. doi: 10.1042/bj1040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet N., Quistorff B., Thieden H. I. Rate-limiting factors in ethanol oxidation by isolated rat-liver parenchymal cells. Effect of ethanol concentration, fructose, pyruvate and pyrazole. Eur J Biochem. 1973 Dec 3;40(1):275–282. doi: 10.1111/j.1432-1033.1973.tb03195.x. [DOI] [PubMed] [Google Scholar]
- Haynes R. C., Jr The fixation of carbon dioxide by rat liver mitochondria and its relation to gluconeogenesis. J Biol Chem. 1965 Oct;240(10):4103–4106. [PubMed] [Google Scholar]
- Krebs H. A., Stubbs M. Factors controlling the rate of alcohol disposal by the liver. Adv Exp Med Biol. 1975;59:149–161. doi: 10.1007/978-1-4757-0632-1_12. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P., Cornell N. W., Krebs H. A. Effect of adenosine on the adenine nucleotide content and metabolism of hepatocytes. Biochem J. 1975 Dec;152(3):593–599. doi: 10.1042/bj1520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel H. G., Reddy W. J., Boshell B. R. The mechanism of inhibition of phosphoenolpyruvate carboxylase by quinolinic acid. Biochim Biophys Acta. 1972 Aug 28;276(2):543–550. doi: 10.1016/0005-2744(72)91015-7. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., van Woerkom G. M., Williamson J. R., Tager J. M. Rate-limiting factors in the oxidation of ethanol by isolated rat liver cells. Biochem J. 1975 Aug;150(2):205–209. doi: 10.1042/bj1500205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp B. V. Rate-limiting steps in ethanol metabolism and approaches to changing these rates biochemically. Adv Exp Med Biol. 1975;56:77–109. doi: 10.1007/978-1-4684-7529-6_4. [DOI] [PubMed] [Google Scholar]
- Ray P. D., Foster D. O., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. IV. Inhibition by L-tryptophan of hepatic gluconeogenesis at the level of phosphoenolpyruvate formation. J Biol Chem. 1966 Sep 10;241(17):3904–3908. [PubMed] [Google Scholar]
- Tischler M. E., Pachence J., Williamson J. R., La Noue K. F. Mechanism of glutamate-aspartate translocation across the mitochondrial inner membrane. Arch Biochem Biophys. 1976 Apr;173(2):448–461. doi: 10.1016/0003-9861(76)90282-4. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Guynn R., Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J. 1972 Apr;127(2):387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneziale C. M., Walter P., Kneer N., Lardy H. A. Influence of L-tryptophan and its metabolites on gluconeogenesis in the isolated, perfused liver. Biochemistry. 1967 Jul;6(7):2129–2138. doi: 10.1021/bi00859a034. [DOI] [PubMed] [Google Scholar]


