Abstract
Animals can learn about sources of danger while minimizing their own risk by observing how others respond to threats. However, the distinct neural mechanisms by which threats are learned through social observation (known as observational fear learning1–4 (OFL)) to generate behavioural responses specific to such threats remain poorly understood. The dorsomedial prefrontal cortex (dmPFC) performs several key functions that may underlie OFL, including processing of social information and disambiguation of threat cues5–11. Here we show that dmPFC is recruited and required for OFL in mice. Using cellular-resolution microendoscopic calcium imaging, we demonstrate that dmPFC neurons code for observational fear and do so in a manner that is distinct from direct experience. We find that dmPFC neuronal activity predicts upcoming switches between freezing and moving state elicited by threat. By combining neuronal circuit mapping, calcium imaging, electrophysiological recordings and optogenetics, we show that dmPFC projections to the midbrain periaqueductal grey (PAG) constrain observer freezing, and that amygdalar and hippocampal inputs to dmPFC opposingly modulate observer freezing. Together our findings reveal that dmPFC neurons compute a distinct code for observational fear and coordinate long-range neural circuits to select behavioural responses.
Social learning is a crucial component of the behavioural repertoire of many species. Learning about threats by observing others helps animals avoid dangerous encounters. However, abnormal OFL can be deleterious; witnessing others experience harrowing events is a common cause of trauma and stressor-related disorders12. Thus, elucidating the neural circuits that underlie OFL has broad implications for understanding how animals learn about threats through observation and for identifying biological risk factors for trauma-related neuropsychiatric illnesses.
In a typical rodent experimental assay for OFL, an observer (OBS) is conditioned to a cue or context by being in proximity to a demonstrator (DEM) that receives cue-shock pairings13,14. During OFL, an OBS integrates social signals conveyed by a threatened DEM with sensory and contextual information about the potential source of the threat. The OBS must also distinguish an observed threat (to other) from one that is directly physically experienced (by self) and consequently generate a behavioural response that minimizes potential harm without compromising other adaptive demands, such as foraging and aiding others. Although prior studies of OFL implicate brain regions involved in empathy and valence assignment, including the caudal anterior cingulate cortex (cACC), mediodorsal thalamus and basolateral amygdala1–3 (BLA), the wider neural circuitry underlying OFL remains unclear.
The dmPFC appears to be uniquely positioned to be a key substrate for OFL. The dmPFC allocates attention when processing ambiguous threat cues to arbitrate and select between competing defensive response options5–11,15. Additionally, dmPFC neurons are engaged by various forms of social cognition, including when differentially representing self and other16–25. These previous findings led us to posit that dmPFC could have a critical role in OFL by forming unique, dynamic representations of observed threats and directing behavioural responses by coordinating dmPFC efferent and afferent activity.
Prefrontal mediation of observed threat
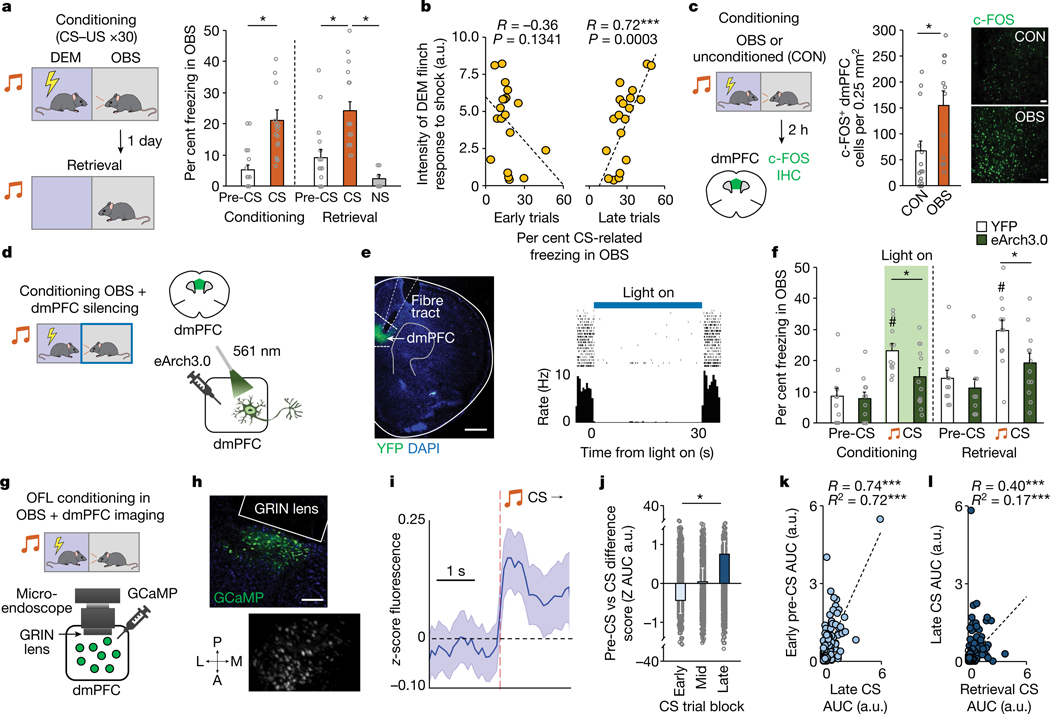
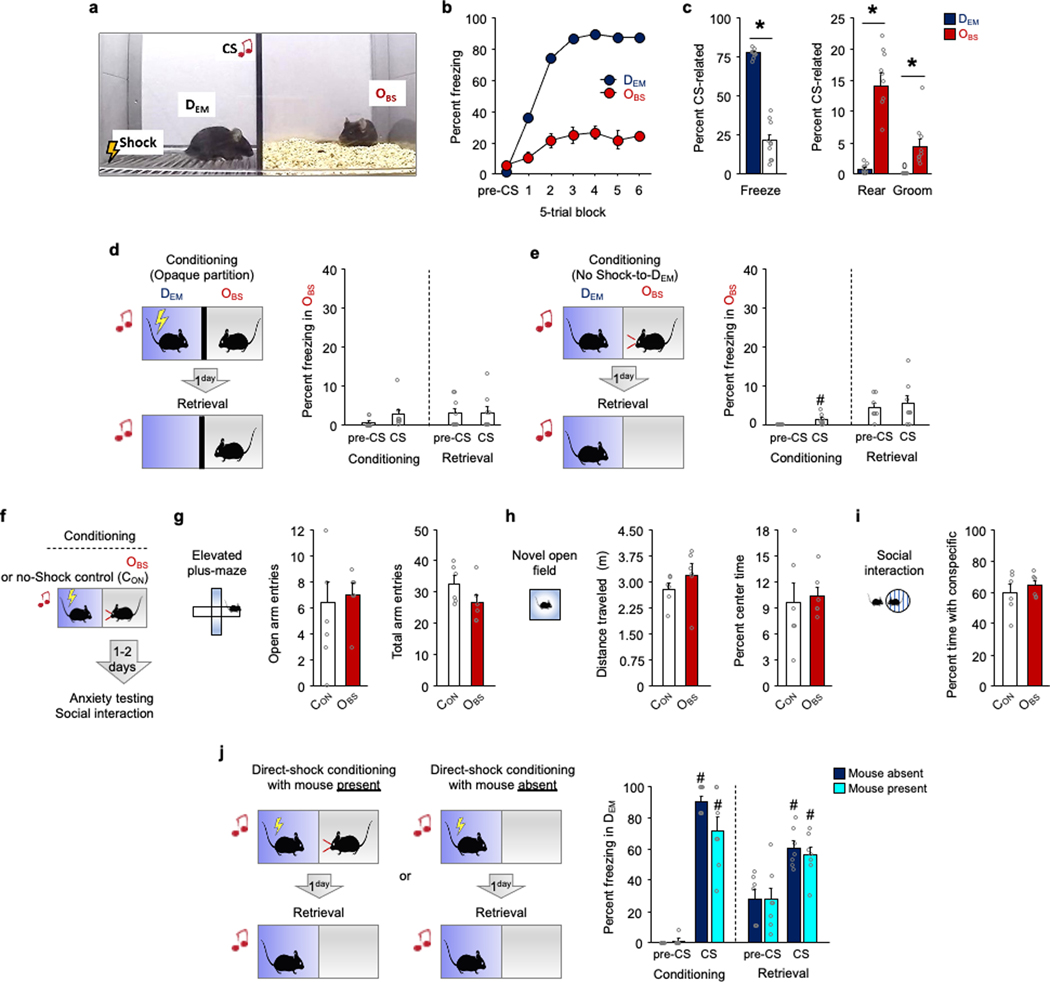
To study the role of dmPFC in OFL, we presented a DEM mouse with 30 pairings of an auditory conditioning stimulus (CS) and unconditioned footshock stimulus (US) while an OBS watched from an adjacent, physically separated compartment. Quantifying freezing as a defensive response in the OBS, we found increased freezing to the CS, relative to pre-CS baseline, during conditioning and a retrieval test performed in the same context (but with the DEM absent) the following day (Fig. 1a and Extended Data Fig. 1a–c). Notably, as in other studies of cued OFL26,27, learning occurred despite OBS–DEM pairs being socially unfamiliar (compare with context in ref. 28), which may reflect the relatively high salience of a discrete cue14. We found that CS-related OBS freezing on late (last ten trials), but not early (first ten trials), conditioning trials correlated with the magnitude of the DEM flinch response to the US, suggesting that observation of the DEM flinch response instructed the association with the CS (Fig. 1b). Also of note, we showed that either removing the shock or preventing the OBS from seeing the DEM was sufficient to prevent cued OFL (Extended Data Fig. 1d,e), as previously reported for contextual OFL28 (see also ref. 21).
Fig. 1 |. Prefrontal mediation of observational fear.
a, Higher OBS CS-related freezing relative to pre-CS during conditioning (*P = 0.0002, n = 17 mice) and relative to pre-CS (*P = 0.0001, n = 17 mice) and a neutral stimulus (*P = 0.0110, n = 7 mice) during retrieval. P values from paired t-tests. NS, novel stimulus. b, DEM shock-induced flinching correlates (Pearson’s r) with OBS freezing on late (last 10), not early (first 10), OFL conditioning trials. n = 17 mice. a.u., arbitrary units. c, There are increased numbers of c-FOS-positive neurons in dmPFC in OBS mice than in CON mice (*P = 0.0077). P value from Bonferroni post hoc tests following mixed-model ANOVA. n = 12–16 mice per group. See Extended Data Fig. 2 for c-FOS-positive neurons in other brain regions. IHC, immunohistochemistry. Scale bars, 100 μm. d, Optogenetic manipulation of dmPFC neurons via eArch3.0 during OFL conditioning. e, Left, eArch3.0–YFP expression and optic fibre tract. Right, effect of eArch3.0 on in vivo dmPFC single-unit activity. Scale bar, 500 μm. f, Optogenetic manipulation of dmPFC neurons during conditioning reduces CS-related OBS freezing relative to YFP controls during conditioning (*P = 0.0351) and light-free retrieval (*P = 0.0399); higher freezing to CS than pre-CS during conditioning (#P = 0.0005) and retrieval (#P = 0.0023) in the YFP group. P values from Bonferroni post hoc tests following mixed-model ANOVA. n = 11 or 12 mice per group. g, Microendoscope dmPFC neuronal calcium imaging during OFL. h, GCaMP7f expression post mortem (top) and in GRIN lens field of-view in vivo (bottom). A, anterior; L, lateral; M, medial; P, posterior. Scale bar, 100 μm. i, Increased dmPFC population calcium activity during CS. n = 8 mice. j, Progressively increasing CS-related calcium activity across early, mid and late OFL conditioning trial blocks (*P = 0.0467). P value from Bonferroni post hoc tests following ANOVA. n = 8 mice. k, Pre-CS calcium activity on Early OFL conditioning trials is related (Pearson’s r ***P < 0.0001, linear regression adjusted R2 ***P < 0.0001) to CS-related activity on late trials. n = 8 mice. l, CS-related activity on late conditioning trials is related (Pearson’s r ***P < 0.0001, linear regression adjusted R2 ***P < 0.0001) to CS-related activity on retrieval. n = 8 mice. Two-tailed statistical tests were used. Data shown as mean ± s.e.m. AUC, area under the curve.
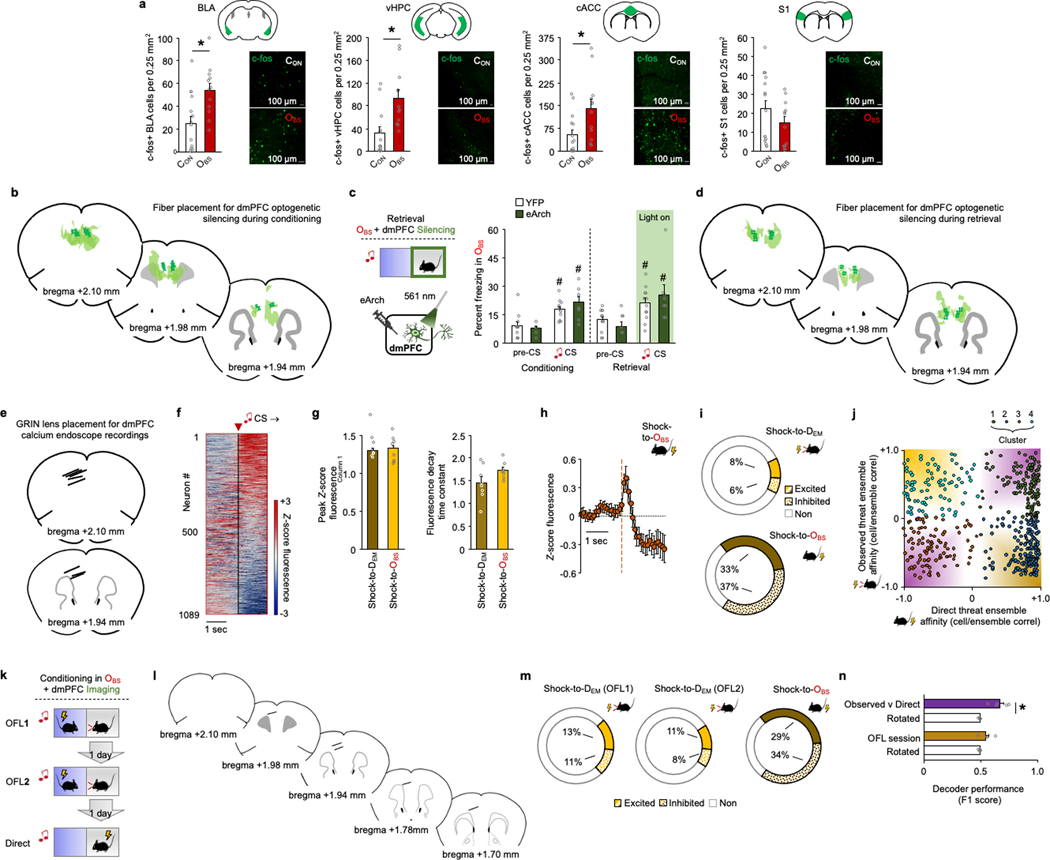
Using this cued-OFL assay, we sought to identify brain regions that are recruited during behaviour by using c-FOS immunochemistry to label OFL-activated neurons. Compared with unconditioned controls (CON), OBS mice had more c-FOS-positive cells in dmPFC, as well as BLA and cACC (consistent with data from other approaches2,27,29–32) and ventral hippocampus (vHPC), but not somatosensory cortex (Fig. 1c and Extended Data Fig. 2a). We next tested the functional involvement of dmPFC in OFL by expressing the inhibitory opsin archaerhodopsin (eArch3.0), and implanted optic fibres in dmPFC to perform in vivo optogenetics to photosilence dmPFC neurons (confirmed by in vivo single-unit recordings) (Fig. 1d,e and Extended Data Fig. 2b). We found that photosilencing dmPFC neurons during each CS–US pairing during OFL conditioning reduced CS-related freezing during both conditioning and retrieval (Fig. 1f). By contrast, dmPFC photosilencing during retrieval CS presentations was without effect (Extended Data Fig. 2c,d), demonstrating that dmPFC is required for formation but—unlike cued direct fear learning33 (DFL)—not for expression of cued OFL; contextual OFL shows a similar dissociation28. However, the question of whether the CS–threat association is formed in dmPFC or, instead, dmPFC relays or instructs the association as it is formed elsewhere (for example, the amygdala) remains to be determined.
Prefrontal coding of observed threat
Given that dmPFC neuronal activity is required for OFL, we next sought to gain insight into the computations performed by dmPFC neurons during OFL by means of cellular-resolution in vivo calcium (Ca2+) imaging in eight freely moving OBS mice. A calcium indicator (GCaMP7f) was expressed in dmPFC neurons (using synapsin (Syn1), a pan-neuronal promoter) along with a microendoscope-attached graded refractive index (GRIN) lens chronically implanted above the transfected area (Fig. 1g,h and Extended Data Fig. 2e). Recordings of 1,089 dmPFC neurons during OFL revealed a small subset of neurons (2%) responsive to CS presentation and showed that CS responsivity increased across OFL conditioning trial blocks as the OBS learned the CS–shock association (Fig. 1i,j and Extended Data Fig. 2f). Of note, we also found that neurons with relatively high pre-CS activity early in OFL exhibited stronger CS-related responses late in OFL, which carried over into retrieval (Fig. 1k,l). This suggests that a non-random subpopulation of basally high-activity dmPFC neurons preferentially encoded the CS as it attained value as a predictor of observational threat.
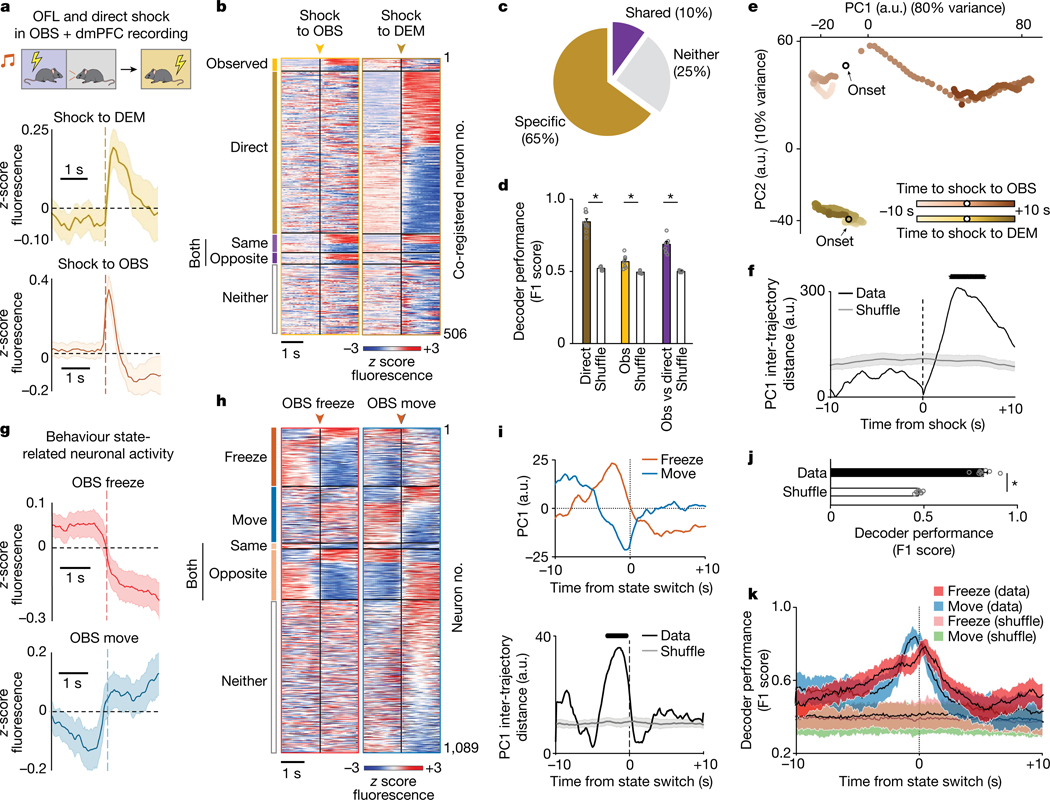
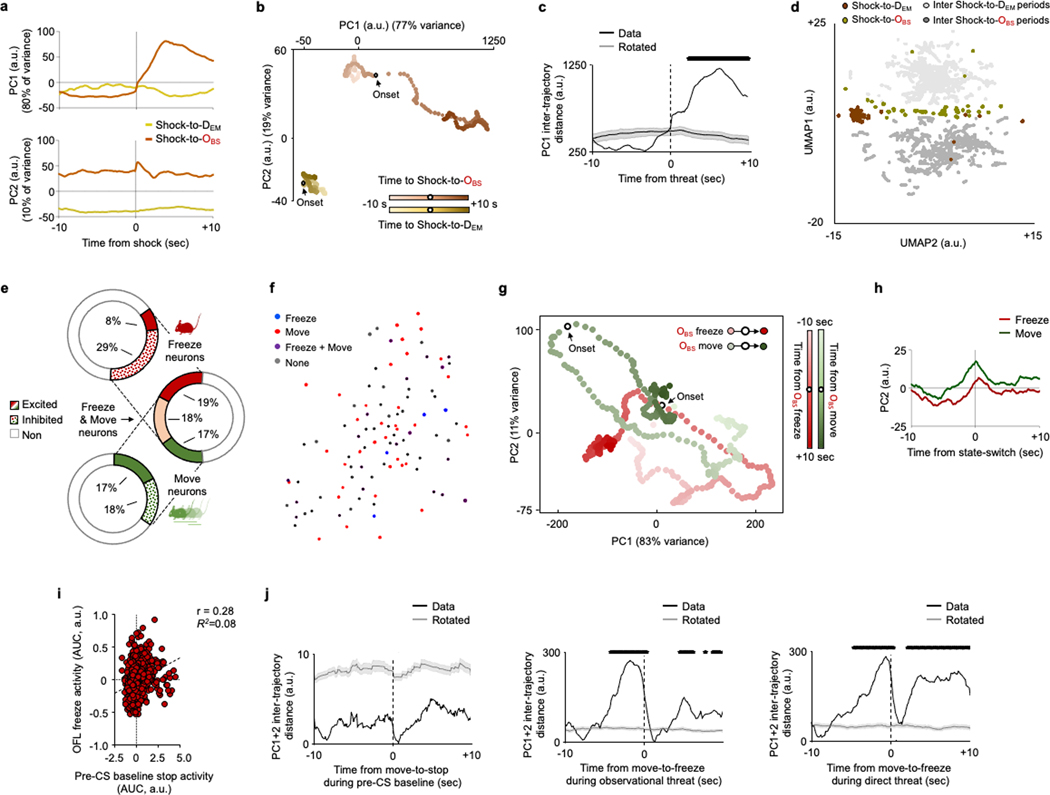
Next, we examined neuronal responses as the OBS watched the DEM receive shocks (Observed) and, in a separate session, subjected the OBS to shock (Direct) to compare the responses of the same dmPFC neurons. Neuronal responses tended to be more prolonged to Observed shock than to Direct shock. Most notably, we identified subpopulations of neurons that responded selectively to a given experience (65% of recorded neurons were experience-responsive, of which 14% specifically responded to Observed shock and 71% to Direct shock). We also found a subset of neurons that were responsive to both types of experience (10%) (Fig. 2a–c and Extended Data Fig. 2g–j). Furthermore, we found that decoders could distinguish neuronal responses to Observed and Direct shocks from shuffled (circularly rotated) data and, crucially, could distinguish between the two forms of experience (Fig. 2d). These data suggest that dmPFC neuronal population activity differentially represents observed and direct experience. In a separate imaging experiment, we found that the population of Observed shock-related neurons was stable across two OFL sessions and that decoders could not discriminate the two episodes, whereas Observed and Direct could be discriminated in the same mice (Extended Data Fig. 2k–n). This indicates that differential dmPFC neuron representation of Observed and Direct shock did not simply reflect a general rule of repeated experience per se.
Fig. 2 |. Prefrontal coding of observed and direct fear.
a, Top, microendoscope imaging of dmPFC neuronal calcium in OBS mice during OFL and direct shock. Increased population calcium activity during observation of shock to the DEM (middle) and direct shock to the OBS (bottom). b, Heat map of calcium activity aligned to shock to the OBS (Observed), Shock to the DEM (Direct), both (same or opposite direction), or neither. c, The percentage of neurons that are responsive to Observed and Direct shock, both, or neither. d, Decoding Observed (Obs) (*P = 0.0014) and Direct (*P = 0.0001) and Observed versus Direct (*P = 0.0001) shock. P values from paired t-tests versus rotated. e, Observed and Direct shock-related activity trajectories occupy distinct places in low-dimensional state space following PCA. f, PC1 trajectory distance aligned to Observed and Direct experience (black line indicates significant difference from shuffle). g, Increased and decreased calcium activity during freeze (top) and move (bottom), respectively. h, Heat map of calcium activity aligned to freeze, move or both (same or opposite direction). i, Top, PC1 neuronal trajectories differentially anticipate upcoming switches to freeze or move state. PC1 trajectory distance is aligned to state switch. j, Decoding freeze and move state (Data) (*P = 0.0001 versus rotated, paired t-test). k, Decoding freeze and move state as a function of time from state switch. P = 0.0001, mixed-model time × group interaction effect. Data shown as mean (e,f,i), mean ± s.e.m. (a,d,g,j) or mean ± s.d. (k). The black line (f,i) denotes a period significantly different from rotated data (Benjamini–Hochberg corrected permutation tests). Two-tailed statistical tests were used. a–k, n = 8 mice.
To further examine the dissociation of observed versus direct experience, we examined the low-dimensional structure of dmPFC neuronal activity using principal components analysis (PCA) and compared the population dynamics during Observed and Direct shock. Approximately 90% of the variance in dmPFC neuronal activity was captured by the two dominant principal components. Notably, the dynamic neuronal trajectories associated with Observed and Direct shock occupied distinct positions in neural state space, even when restricting the analysis to a subset of ten trials in which freezing levels were similar in the two conditions (Fig. 2e,f and Extended Data Fig. 3b,c). Similar results were obtained using a non-linear dimensionality reduction method, uniform manifold approximation and projection (UMAP) (Extended Data Fig. 3d). Together, these data suggest that dmPFC neuronal population activity differentially represents Observed and Direct experience. Whether the dmPFC population coding for Observed experience per se is in and of itself sufficient to support OFL and/or learning about Direct experience remains to be determined. Doing so will be challenging given that current approaches, such as FosTRAP (Fos-dependent targeted recombination in active populations), cannot isolate the OFL-only population from the small subpopulation of neurons that co-code for Observed and Direct experience.
Prefrontal encoding of state transitions
We next tested whether dmPFC neurons also code for OBS behaviour during OFL. We found spatially intermingled neurons exhibiting activity as the OBS transitioned from a freezing-to-moving or a moving-to-freezing state during OFL (Fig. 2g,h and Extended Data Fig. 3e,f). Some neurons responded exclusively to one form of behavioural state transition, whereas others were active during either transition-type but in opposite directions. These data show, first, that dmPFC neuronal activity codes for specific OBS behavioural states, echoing recent findings from other fear and social behaviour paradigms9,18,34,35 and second, that some neurons co-code for opposing behaviours with opposing activity patterns—a feature that could support rapid switching between opposing behavioural states. Neuronal activity related to OBS moving-to-freezing transitions during OFL did not reflect the concomitant behaviour state of the DEM (fewer than 2% of neurons responded to DEM freeze–move state and, of those, fewer than 1.5% were co-responsive to OBS state). Thus, it appears that only certain types of DEM behaviour—specifically those that are most instructive of OFL (shock-related flinching, see Fig. 1b)—are coded in dmPFC neurons (see also ref. 36).
To gain more insight into dmPFC neuron population activity associated with OBS behaviour state, we decomposed neural activity using PCA and examined the dynamics of the two components capturing 94% of the variance. We found that these two components each reflected a different feature of OBS behaviour. Principal component 2 (PC2) values increased at switching, suggesting a representation related to response execution, whereas PC1 values changed prior to a switch between behavioural states, with increased and decreased activity before transitions to freeze and move, respectively (Fig. 2i and Extended Data Fig. 3g,h). These pre-switch dynamics suggest dmPFC neuronal representations of behavioural response selection. Indeed, decoders could differentiate upcoming OBS freeze–move state transitions based on dmPFC neuronal activity in the seconds preceding transitions (Fig. 2j,k). We also found increased OBS dmPFC neuronal activity preceding moving-to-freezing transitions during DFL, but not preceding moving-to-stopping transitions during pre-conditioning baseline (Extended Data Fig. 3i,j). This indicates a role for dmPFC neurons in behaviour state switching in fearful mice, but not in locomotor switches per se. However, given that only a minority (15–18%) of neurons encoded transitions to Observed and Direct experience in the same mice, state switching during each experience engages different dmPFC populations.
Functionally dissociable cortical output
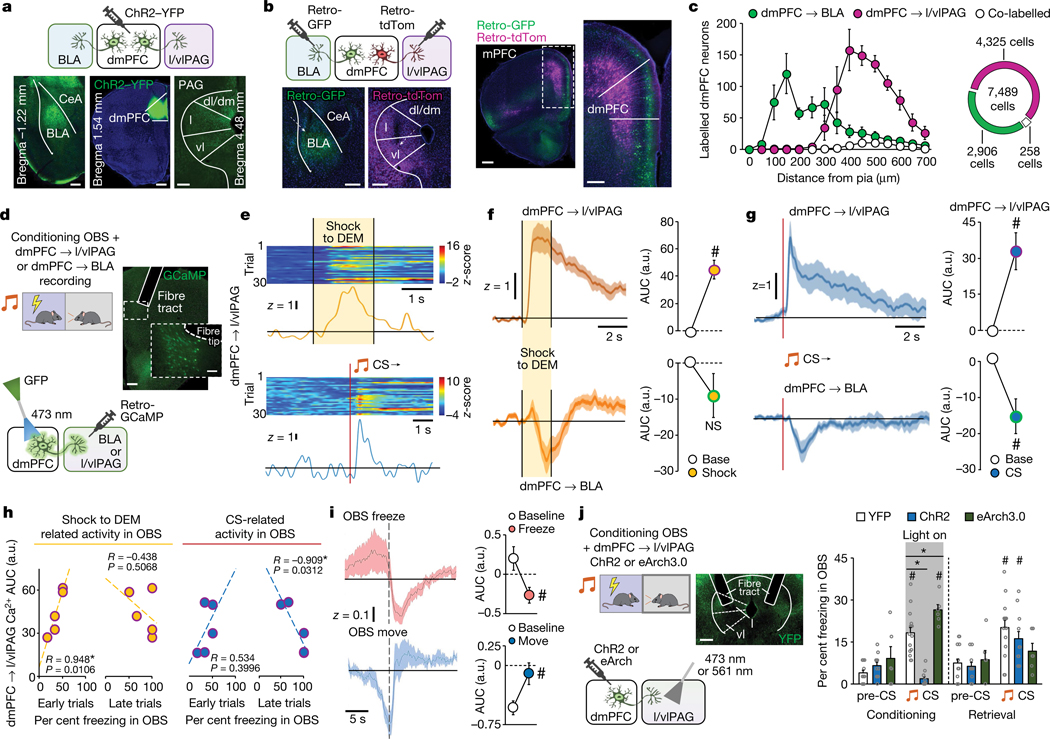
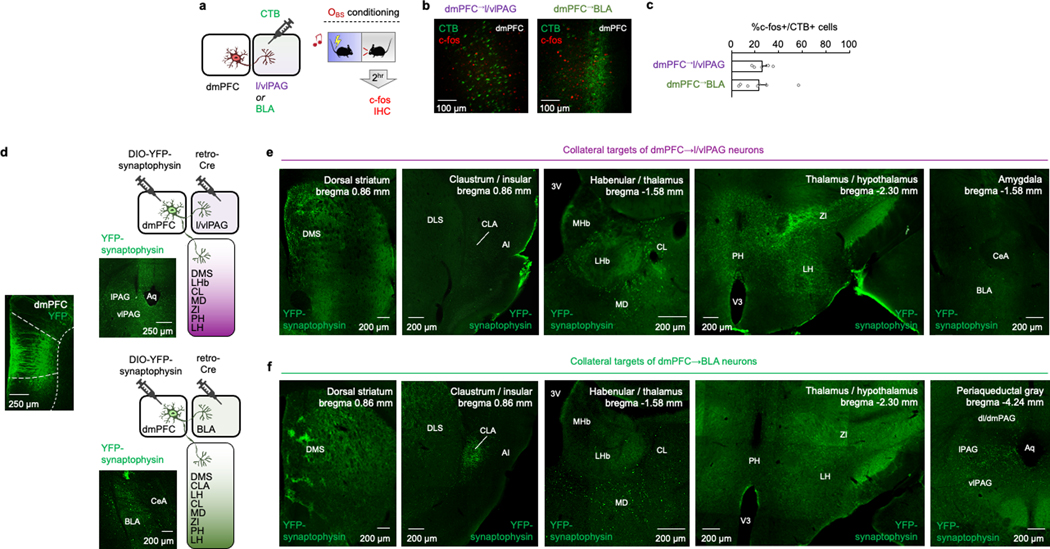
We reasoned that the capacity of dmPFC neurons to code for multiple components of OFL, including the selection of distinct behavioural responses, could in part reflect differential contributions of distinct dmPFC projection populations. Two dmPFC projection targets, the lateral and ventrolateral PAG (l/vlPAG) and BLA (Fig. 3a), are known to exhibit opposing responses to punished conflict and differentially regulate multiple processes relevant to OFL, including choice selection, social stress regulation, defensive behaviour, and direct and observational fear encoding37–43. We found that following OFL, around 25% of cholera toxin B (CTB)-labelled dmPFC neurons projecting to l/vlPAG or BLA were activated (that is, c-FOS-positive) (Extended Data Fig. 4a–c). Injecting retrograde neuronal viral tracers into l/vlPAG and BLA showed that dmPFC neurons projecting to the two regions concentrated in the superficial and deep layers of the cortex, respectively, but with minimal tracer co-expression in the same neurons (Fig. 3b,c). However, using anterograde synaptophysin virus neuronal tracing to separately label collateral targets of l/vlPAG- and BLA-projecting dmPFC neurons indicated that both populations targeted some similar areas in striatum and thalamus (Extended Data Fig. 4d–f). Thus, although the dmPFC→l/vlPAG and dmPFC→BLA pathways are largely anatomically discrete from one another, they could broadcast to some common forebrain areas.
Fig. 3 |. Prefrontal–midbrain circuit modulation of observational fear.
a, ChR2-labelled dmPFC axons in BLA and l/vlPAG. CeA, central amygdala; dm, dorsomedial PAG; dl, dorsolateral PAG; l, lateral PAG; vl, ventrolateral PAG. Scale bars, 200 μm, 500 μm and 200 μm (left to right). b, Retrograde virus-labelled dmPFC neuronal projections to BLA and l/vlPAG (arrows depict injector tip). mPFC, medial prefrontal cortex. Scale bars, 200 μm, 200 μm, 500 μm and 100 μm (left to right). c, Non-overlapping dmPFC neuronal projections. P = 0.0001 mixed-model time × group interaction effect. n = 5 mice. d, Right, fibre photometry calcium imaging of dmPFC→BLA or dmPFC→l/vlPAG neurons. Left, GCaMP expression and fibre tip and tract position. Scale bars, 200 μm (main image) and 50 μm (magnified view).e, Example trace and heat map of dmPFC→l/vlPAG neuronal activity during shock to DEM (top) and CS presentation (bottom). f, Differential shock to DEM population calcium activity in dmPFC→l/vlPAG (#P = 0.0037, paired t-test, n = 5 mice) and dmPFC→BLA neurons (n = 6 mice). g, Differential CS-related population calcium activity in dmPFC→l/vlPAG (#P = 0.0202, n = 5 mice) and dmPFC→BLA neurons (#P < 0.0307, n = 6 mice). P values from paired t-tests. h, Left, shock to DEM activity correlates (Pearson’s r) with OBS freezing on early (first 5) OFL conditioning trials. Right, CS-related activity correlates (Pearson’s r) with OBS freezing on late (last 5) trials. i, Differential freeze (#P = 0.0035) and move (#P = 0.0344) population calcium activity in dmPFC→l/vlPAG neurons. P values from paired t-tests. n = 5 mice. j, Optogenetic manipulation of dmPFC→l/vlPAG axons bidirectionally alters CS-related OBS freezing during conditioning (ChR2 *P < 0.0001, n = 11; eArch3.0, *P = 0.0088, n = 6), versus YFP controls (n = 15). Higher CS-related freezing versus pre-CS during conditioning in YFP (#P < 0.0001) and eArch3.0 (#P = 0.0040), and lower freezing in ChR2 (#P = 0.0056). Higher CS-related freezing versus pre-CS during light-free retrieval in YFP (#P = 0.0341) and ChR2 (#P = 0.0053), but not eArch3.0. P values from Bonferroni post hoc tests following mixed-model ANOVA. Two-tailed statistical tests were used. Data shown as mean ± s.e.m. Scale bar, 200 μm.
Next, to examine the functional roles of the dmPFC→l/vlPAG and dmPFC→BLA populations in OFL, we used in vivo fibre photometry to measure calcium activity in these neurons (Fig. 3d and Extended Data Fig. 5a,b). Replicating our single-neuron imaging data, we found increased neuronal activity as OBS mice watched DEM mice being shocked but, crucially, only in dmPFC→l/vlPAG neurons and not in dmPFC→BLA neurons (Fig. 3e,f). The two pathways also had differing CS-related responses: dmPFC→l/vlPAG neurons showed increased activity during CS presentation, whereas dmPFC→BLA neuronal activity was decreased (Fig. 3g). Moreover, we found that OBS freezing correlated with responses during Shock to the DEM on early OFL trials but, in the same neurons, correlated with CS-related dmPFC→l/vlPAG neuronal responses on late OFL trials (Fig. 3h and Extended Data Fig. 5c). This shift in the relationship between dmPFC→l/vlPAG activity and OBS freezing across trials suggests that dmPFC→l/vlPAG neurons report the dynamic transfer of information about the observed threat to the CS as the CS–threat association is formed.
Another notable finding from these analyses was that larger CS-related responses in dmPFC→l/vlPAG neurons corresponded to less freezing in OBS mice. Accordingly, when we temporally aligned the activity of dmPFC→l/vlPAG neurons to OBS freezing, we found that the onset of freezing bouts corresponded to a decrease in dmPFC→l/vlPAG activity, which reversed upon re-initiation of movement (Fig. 3i and Extended Data Fig. 5d,e). Moreover, we found that optogenetic manipulation of dmPFC→l/vlPAG neurons via ChR2 or eArch3.0 decreased or increased, respectively, CS-related freezing during OFL (Fig. 3j). In the case of eArch3.0, CS-related freezing was also decreased on light-free retrieval, indicating a role for dmPFC→l/vlPAG neurons in consolidating OFL. These data suggest that dmPFC→l/vlPAG neurons oppose freezing, a finding broadly in line with evidence that this pathway inhibits affective pain behaviour44 and suppresses contextual DFL freezing45. Echoing this latter finding, we found that ChR2 activation decreased DFL CS-related freezing (Extended Data Fig. 5f–h). On the basis of these data, we posit that whereas dmPFC neurons differentially represent Observed and Direct experience, engagement of dmPFC output to l/vlPAG opposes freezing or promotes movement during different forms of threat.
Functionally opposing cortical inputs
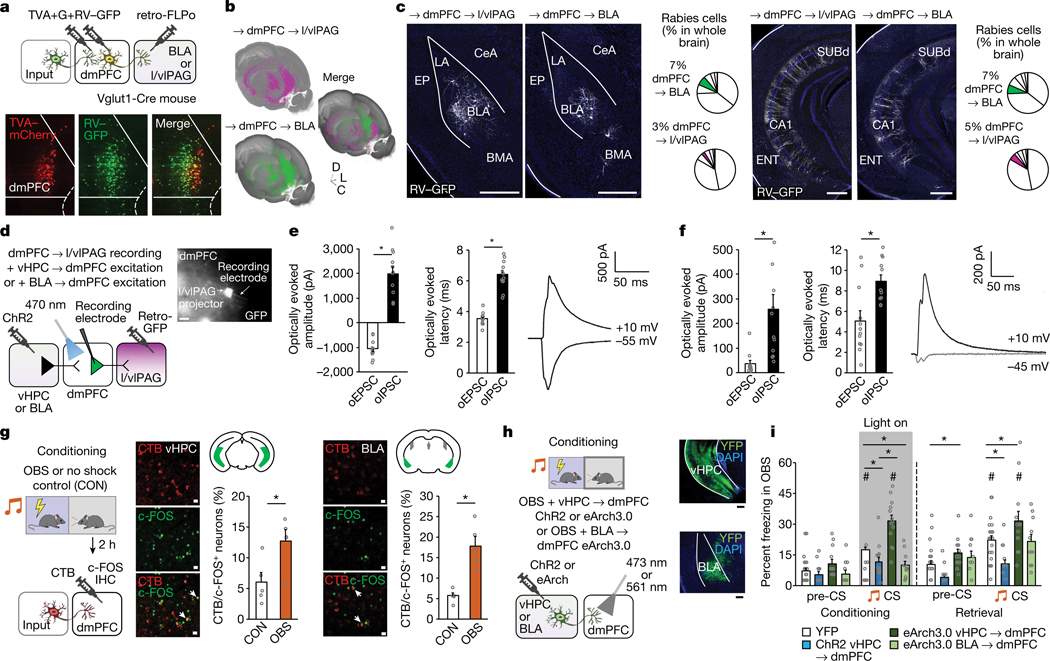
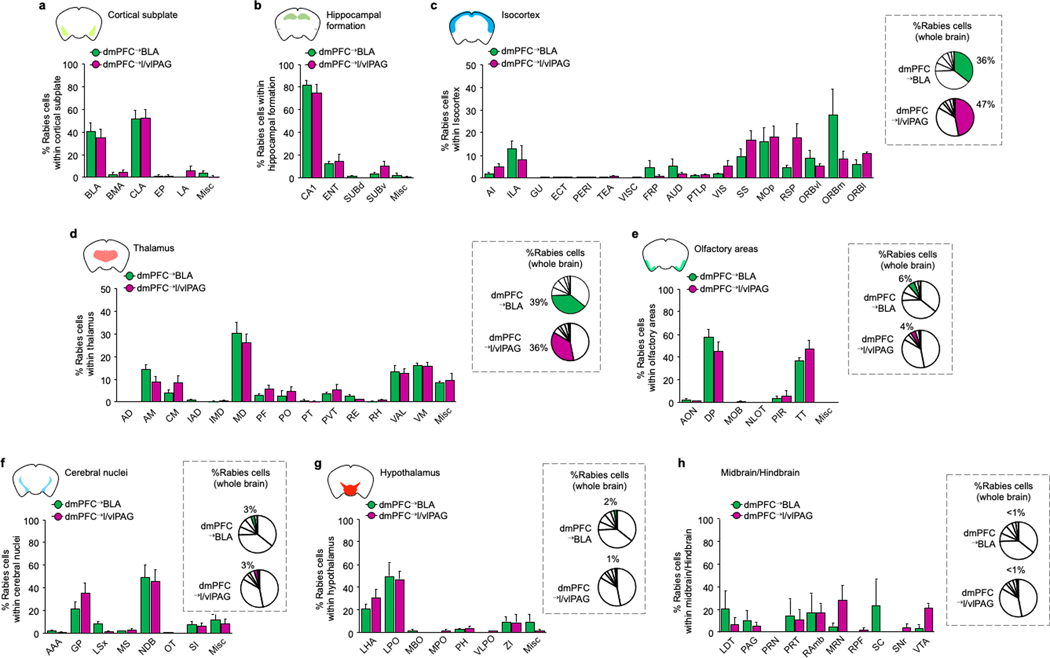
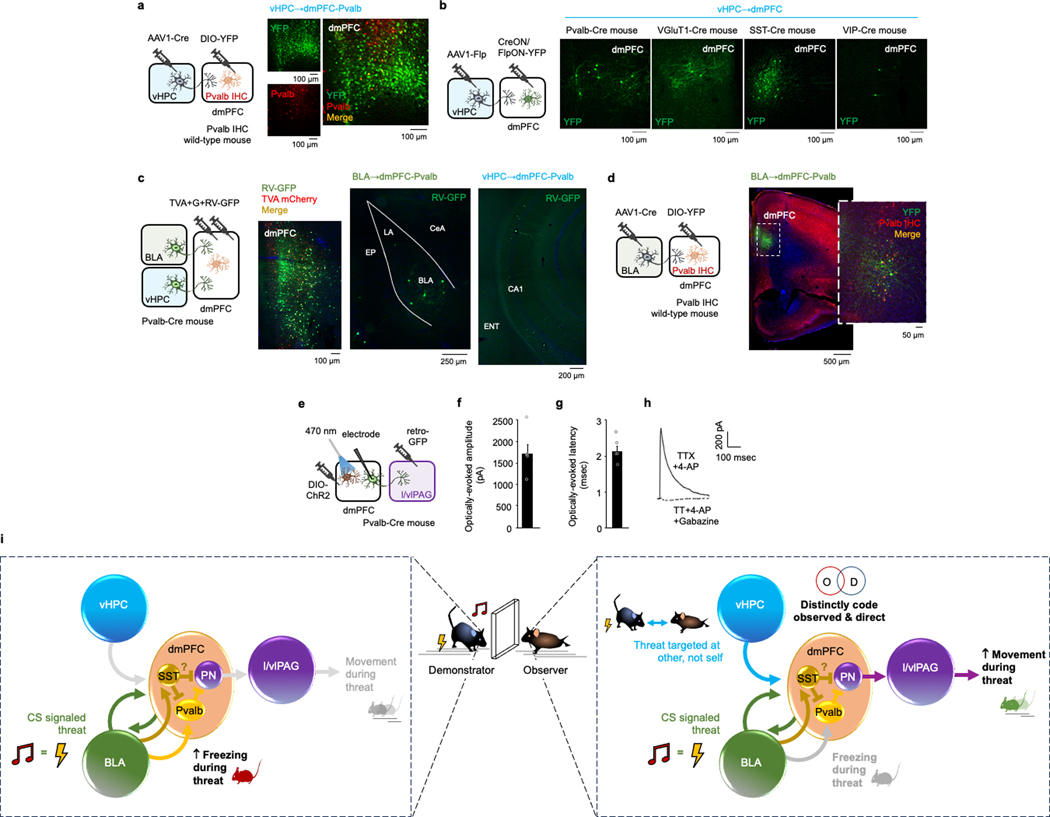
Our findings so far demonstrate that dmPFC neurons distinctly code for OFL and regulate behavioural state in part via output to the l/vlPAG. These data do not, however, identity the inputs that modulate the ability of dmPFC to perform these functions. Using transsynaptic rabies virus tracing, we found that dmPFC→l/vlPAG (and dmPFC→BLA) neurons receive monosynaptic input from distal brain regions (Fig. 4a,b and Extended Data Figs. 6–8), including two regions we had found to be activated by OFL (Fig. 1d): BLA (primarily the rostral part) and vHPC (Fig. 4c). We confirmed these inputs were functional using in vitro ChR2-assisted circuit mapping of vHPC inputs to show photoexcitation of either vHPC or BLA axons in dmPFC generated postsynaptic responses in dmPFC→l/vlPAG neurons identified by GFP expression (Fig. 4d–f). These data showed that photoexcitation of vHPC input produced excitatory and inhibitory responses in dmPFC→l/vlPAG neurons, whereas BLA excitation predominantly produced disynaptic feedforward inhibition. This effect could be owing to BLA activation of parvalbumin-expressing interneurons (Pvalb-IN), given that BLA neurons innervate dmPFC Pvalb-INs46 and photoexciting dmPFC Pvalb-INs inhibits dmPFC→l/vlPAG neurons (Extended Data Fig. 9a–h). Thus, vHPC and BLA inputs are positioned to differentially modulate dmPFC and its output to l/vlPAG. Furthermore, by exerting inhibitory control over dmPFC→l/vlPAG activity and behavioural responses mediated by these neurons, BLA modulation of dmPFC Pvalb-INs could privilege freezing or moving, depending on the nature (observed or direct) of the threat experienced (Extended Data Fig. 9i).
Fig. 4 |. In vitro and in vivo analyses of vHPC and BLA inputs to dmPFC→l/vlPAG neurons.
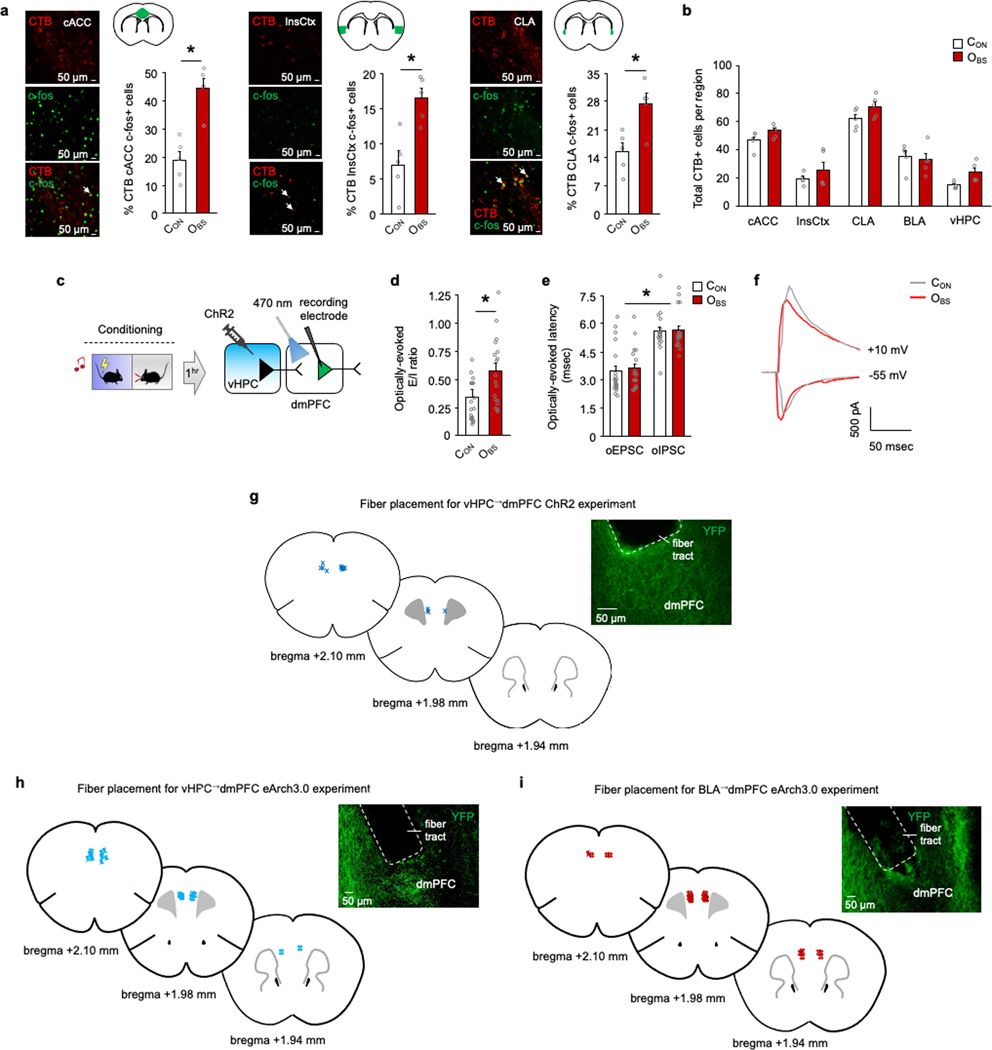
a, Top, intersectional rabies virus (RV) labelling of transynaptic inputs to dmPFC→l/vlPAG and dmPFC→BLA neurons. Bottom, TVA–mCherry (TVA) and RV–GFP expression. G, glycoprotein. b, Three-dimensional pseudocoloured depiction of RV expression in dmPFC→l/vlPAG and dmPFC→BLA neurons. C, caudal; D, dorsal; L, lateral. c, RV labelling and whole-brain percentage of RV-labelled neurons in BLA and vHPC. n = 5–7 mice per group. BMA, basomedial amygdala; ENT, entorhinal cortex; EP, endopiriform; LA, lateral amygdala; SUBd, subiculum dorsal part. Scale bars, 500 μm. d, Left, ChR2-assisted mapping of vHPC or BLA inputs to dmPFC→l/vlPAG neurons. Right, GFP-labelled dmPFC→l/vlPAG neuron proximal to recording electrode. Scale bar, 10 μm e, Optically evoked vHPC-mediated inhibitory postsynaptic current (oIPSC) and excitatory postsynaptic current (oEPSC) amplitude (*P = 0.0001) and latency (*P = 0.0001) in dmPFC→l/vlPAG neurons, with example traces. P values from paired t-tests. n = 12 cells, N = 5 mice. f, Optically evoked BLA-mediated oIPSC and oEPSC amplitude (P = 0.0021) and latency (P = 0.0021) in dmPFC→l/vlPAG neurons, with example traces. P values from paired t-tests. n = 12 cells, N = 5 mice. g, Increased number of c-FOS-positive neurons after OFL relative to CON in dmPFC-projecting vHPC (*P = 0.0244) and BLA (*P = 0.0010) cells. P values from paired t-tests. n = 5 mice per group. Scale bars, 50 μm. h, Left, optogenetic manipulation of vHPC→dmPFC (ChR2 and eArch3.0) or BLA→dmPFC (eArch3.0) axons. Right, virus expression. Scale bars, 200 μm. i, Optogenetic manipulation of vHPC→dmPFC axons bidirectionally alters CS-related freezing (right) during conditioning (ChR2 *P = 0.0241, eArch3.0 *P < 0.0001) and retrieval (ChR2 *P = 0.0033, eArch3.0 *P = 0.0095); eArch3.0 manipulation of BLA→dmPFC decreases CS-related freezing during conditioning (*P = 0.0135), versus YFP controls. Higher CS-related freezing versus pre-CS in vHPC→dmPFC-eArch3.0 group (conditioning, #P < 0.0001, retrieval #P = 0.0052) and YFP controls (conditioning, #P < 0.0001, retrieval #P < 0.0001) but not in vHPC→dmPFC-ChR2 or BLA→dmPFC-eArch3.0 group. P values from Bonferroni post hoc tests following mixed-model ANOVA. n = 7–32 mice per group. Two-tailed statistical tests were used. Data shown as mean ± s.e.m.
To confirm a role for BLA and vHPC inputs in OFL, we combined CTB retrograde neuronal tracing with c-FOS immunochemistry to show that OBS mice had more CTB/c-FOS-positive double-labelled dmPFC-projecting neurons than CON mice in vHPC and BLA, among other regions (Fig. 4g and Extended Data Fig. 10a,b). Then, using ex vivo neuronal recordings, we demonstrated that OFL caused lasting synaptic modifications in vHPC neurons innervating the dmPFC (Extended Data Fig. 10c–f). Finally, we used in vivo optogenetics to assess the effects of manipulating ChR2 or eArch3.0-transfected vHPC axons, or eArch3.0-transfected BLA axons, in dmPFC during OFL conditioning (Fig. 4h and Extended Data Fig. 10g–i). Light manipulation of ChR2-expressing and eArch3.0-expressing vHPC→dmPFC neurons decreased or increased CS-related OBS freezing on conditioning and light-free retrieval, respectively (Fig. 4i). Conversely, light shone on eArch3.0-expressing BLA→dmPFC axons decreased CS-related OBS freezing on conditioning, in line with findings in non-OFL fear assays9,47–49 (Fig. 4i). These data show that vHPC inputs constrain, whereas BLA inputs promote, OBS freezing—the latter potentially involving a BLA–dmPFC–BLA feedback loop50. More generally, these findings begin to uncover how dmPFC integrates information from competing upstream inputs, thereby enriching the coding of observational fear and subsequent selection of behavioural responses via output to l/vlPAG and other structures.
Conclusion
Elucidating the neural basis of learning by observing others has broad implications for understanding how animals, including humans, recognize and effectively respond to threat. Our findings reveal that the dmPFC has a key role in determining how discrete environmental cues become associated with threat solely through observation of the behaviour of a conspecific in the presence of those cues. We find that dmPFC neuronal population dynamics perform computations to code the presence of a threat detected from observing the behaviour of another mouse behaviour and distinguish this from the direct experience. Thus, population-level coding by dmPFC neurons could provide a mechanism through which danger to self versus other is parsed to enable the selection of behavioural strategies specific to the type of threat encountered.
Our data also show how the dmPFC is positioned as a neural hub that coordinates long-range circuit inputs from the amygdala and hippocampus and outputs to the midbrain PAG that serve to calibrate behavioural responses in the OBS. Of note, neuronal pathways opposing freezing to an observed threat may enable animals to engage in adaptive behavioural strategies, such as risk assessment and comforting of others19. In this way, an important function of the dmPFC during observation of threat may be to balance harm mitigation with the need to fulfil other ongoing essential survival functions. Our findings also suggest that maladaptive responses to socially learned threat could arise in part from deficits in dmPFC and its interacting circuits and contribute to neuropsychiatric disorders from psychopathy51 to trauma and stressor-related disorders52.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-023-07008-1.
Extended Data
Extended Data Fig. 1 |.
OFL-related behavioural experiments
Extended Data Fig. 2 |.
OFL-related neuronal activation and in vivo optogenetic manipulations
Extended Data Fig. 3 |.
In vivo single cell resolution calcium recordings in dmPFC during OFL
Extended Data Fig. 4 |.
OFL-related neuronal activation, dmPFC“M/vlPAG or dmPFC-^BLA collaterals
Extended Data Fig. 5 |.
Fibre photometry calcium recordings and optogenetic manipulations
Extended Data Fig. 6 |.
Rabies virus labelling of inputs to dmPFC neurons
Extended Data Fig. 7 |.
Quantification of rabies virus-labelling of inputs to dmPFC neurons
Extended Data Fig. 8 |.

Rabies virus control experiments
Extended Data Fig. 9 |.
Input-Output analysis of dmPFC-Pvalb neurons
Extended Data Fig. S10 |.
Analyses of dmPFC inputs
Supplementary Material
References
- 1.Kondrakiewicz K, Kostecki M, Szadzinska W & Knapska E. Ecological validity of social interaction tests in rats and mice. Genes Brain Behav. 18, e12525 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Olsson A, Knapska E. & Lindstrom B. The neural and computational systems of social learning. Nat. Rev. Neurosci 21, 197–212 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Burgos-Robles A, Gothard KM, Monfils MH, Morozov A. & Vicentic A. Conserved features of anterior cingulate networks support observational learning across species. Neurosci. Biobehav. Rev 107, 215–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keum S. & Shin HS Rodent models for studying empathy. Neurobiol. Learn. Mem 135, 22–26 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Blanchard DC, Griebel G, Pobbe R. & Blanchard RJ Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev 35, 991–998 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Qi S. et al. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl Acad. Sci. USA 115, 3186–3191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanselow MS Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev 1, 429–438 (1994). [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE & Pine DS Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry 173, 1083–1093 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Jercog D. et al. Dynamical prefrontal population coding during defensive behaviours. Nature 595, 690–694 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Mobbs D. The ethological deconstruction of fear(s). Curr. Opin. Behav. Sci 24, 32–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe MJ & Killcross S. Modulation of attention and action in the medial prefrontal cortex of rats. Psychol. Rev 125, 822–843 (2018). [DOI] [PubMed] [Google Scholar]
- 12.DSM-5. Diagnostic and Statistical Manual of Mental Disorders, 4th edn (APA Press, 2013). [Google Scholar]
- 13.Kim A, Keum S. & Shin HS Observational fear behavior in rodents as a model for empathy. Genes Brain Behav. 18, e12521 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Preston SD & de Waal FB Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–20 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Cummings KA & Clem RL Prefrontal somatostatin interneurons encode fear memory. Nat. Neurosci 23, 61–74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yizhar O. & Levy DR The social dilemma: prefrontal control of mammalian sociability. Curr. Opin. Neurobiol 68, 67–75 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Chen P. & Hong W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang ,B. et al. Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron 100, 700–714.e709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu YE et al. Neural control of affiliative touch in prosocial interaction. Nature 599, 262–267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padilla-Coreano N. et al. Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature 603, 667–671 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito W, Palmer AJ & Morozov A. Social synchronization of conditioned fear in mice requires ventral hippocampus input to the amygdala. Biol. Psychiatry 93, 322–330 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheggia D. et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat. Neurosci 23, 47–60 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Piva M. et al. The dorsomedial prefrontal cortex computes task-invariant relative subjective value for self and other. eLife 8, e44939 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F. et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Murugan M. et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e1616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yusufishaq S. & Rosenkranz JA Post-weaning social isolation impairs observational fear conditioning. Behav. Brain Res. 242, 142–149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allsop SA et al. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342.e1318 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon D. et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci 13, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keum S. & Shin HS Neural basis of observational fear learning: a potential model of affective empathy. Neuron 104, 78–86 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Paradiso E, Gazzola V. & Keysers C. Neural mechanisms necessary for empathy-related phenomena across species. Curr. Opin. Neurobiol 68, 107–115 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Smith ML, Asada N. & Malenka RC Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillinger C, Yalcin I, Barrot M. & Veinante P. Efferents of anterior cingulate areas 24a and 24b and midcingulate areas 24a′ and 24b′ in the mouse. Brain Struct. Funct 223, 1747–1778 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Sierra-Mercado D, Padilla-Coreano N. & Quirk GJ Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halladay LR & Blair HT Distinct ensembles of medial prefrontal cortex neurons are activated by threatening stimuli that elicit excitation vs. inhibition of movement. J Neurophysiol. 114, 793–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padilla-Coreano N, Tye KM & Zelikowsky M. Dynamic influences on the neural encoding of social valence. Nat. Rev. Neurosci 23, 535–550 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z. et al. Ventromedial prefrontal neurons represent self-states shaped by vicarious fear in male mice. Nat. Commun 14, 3458 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin TB et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat. Neurosci 20, 260–270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siciliano CA et al. A cortical–brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vander Weele CM et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assareh N, Bagley EE, Carrive P. & McNally GP Brief optogenetic inhibition of rat lateral or ventrolateral periaqueductal gray augments the acquisition of Pavlovian fear conditioning. Behav. Neurosci 131, 454–459 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Courtin J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Ozawa T. et al. A feedback neural circuit for calibrating aversive memory strength. Nat. Neurosci 20, 90–97 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Haaker J, Yi J, Petrovic P. & Olsson A. Endogenous opioids regulate social threat learning in humans. Nat. Commun 8, 15495 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J. et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nat. Neurosci 22, 1659–1668 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Rozeske RR et al. Prefrontal–periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron 97, 898–910.e896 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Anastasiades PG, Marlin JJ & Carter AG Cell-type specificity of callosally evoked excitation and feedforward inhibition in the prefrontal cortex. Cell Rep. 22, 679–692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senn V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E. & Quirk GJ Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagihara KM et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature 594, 403–407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGarry LM & Carter AG Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J. Neurosci 36, 9391–9406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson SW, Bechara A, Damasio H, Tranel D. & Damasio AR Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci 2, 1032–1037 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Shin LM, Rauch SL & Pitman RK Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. NY Acad. Sci 1071, 67–79 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.