Abstract
When cultivated together with pieces of cartilage biosynthetically labelled with 35S in their proteoglycans, rabbit macrophages, differentiated in vitro from bone-marrow cells, cause the release of soluble 35S-labelled material into the culture medium. This process is inhibited by killing the macrophages or by cycloheximide treatment, and is due to the secretion by the cells of a metal-dependent neutral proteinase capable of degrading cartilage proteoglycan subunits into fragments of high molecular weight. Enzyme activity is optimum at about pH7, and is inhibited by EDTA, o-phenanthroline, cysteine or serum, but not by di-isopropyl phosphorofluoridate nor by 4-hydroxymercuribenzoate. The effect of EDTA is partially reversed by Co2+ or Zn2+ ions. The enzyme is eluted from Sephadex G-150 columns as a single peak of material (apparent mol.wt. 17000) that contains also most of the proteolytic activity exerted by culture media on Azocoll (denatured collagen) or on casein. The possible role of this metalloproteinase in chronic inflammatory processes is discussed, particularly in connection with joint erosions in rheumatoid arthritis.
Full text
PDF




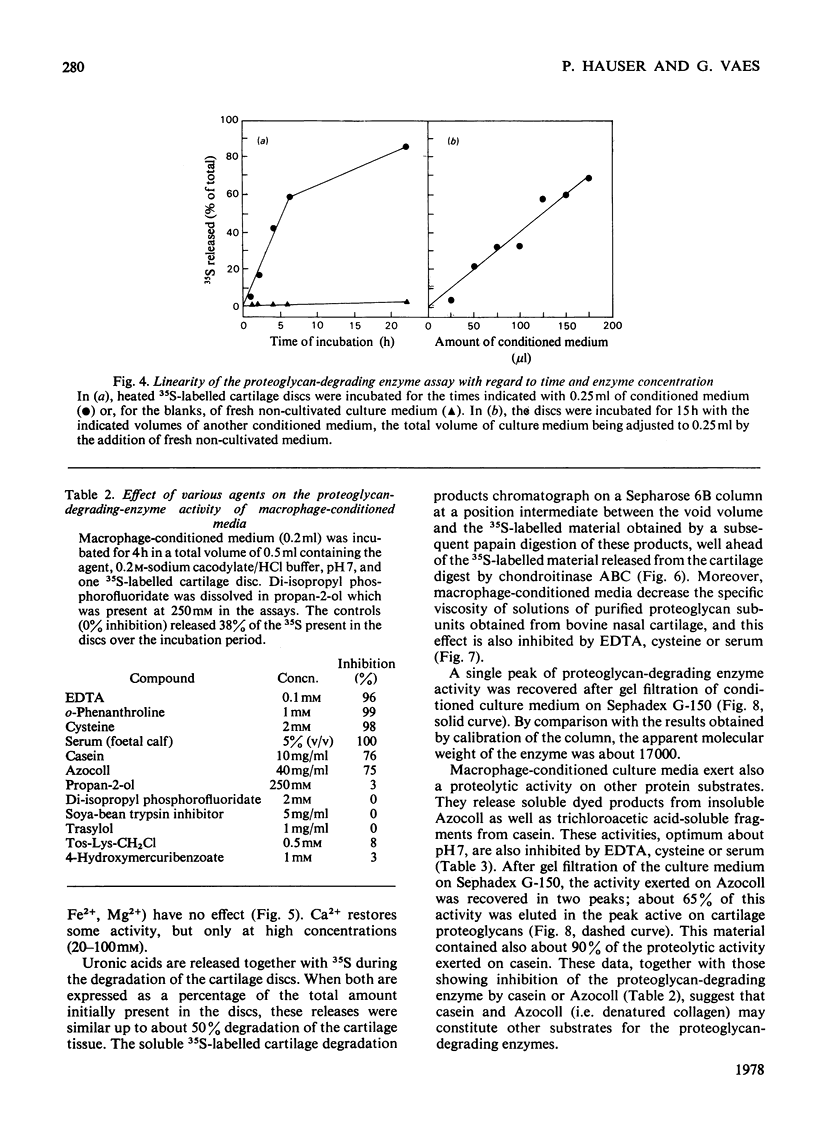
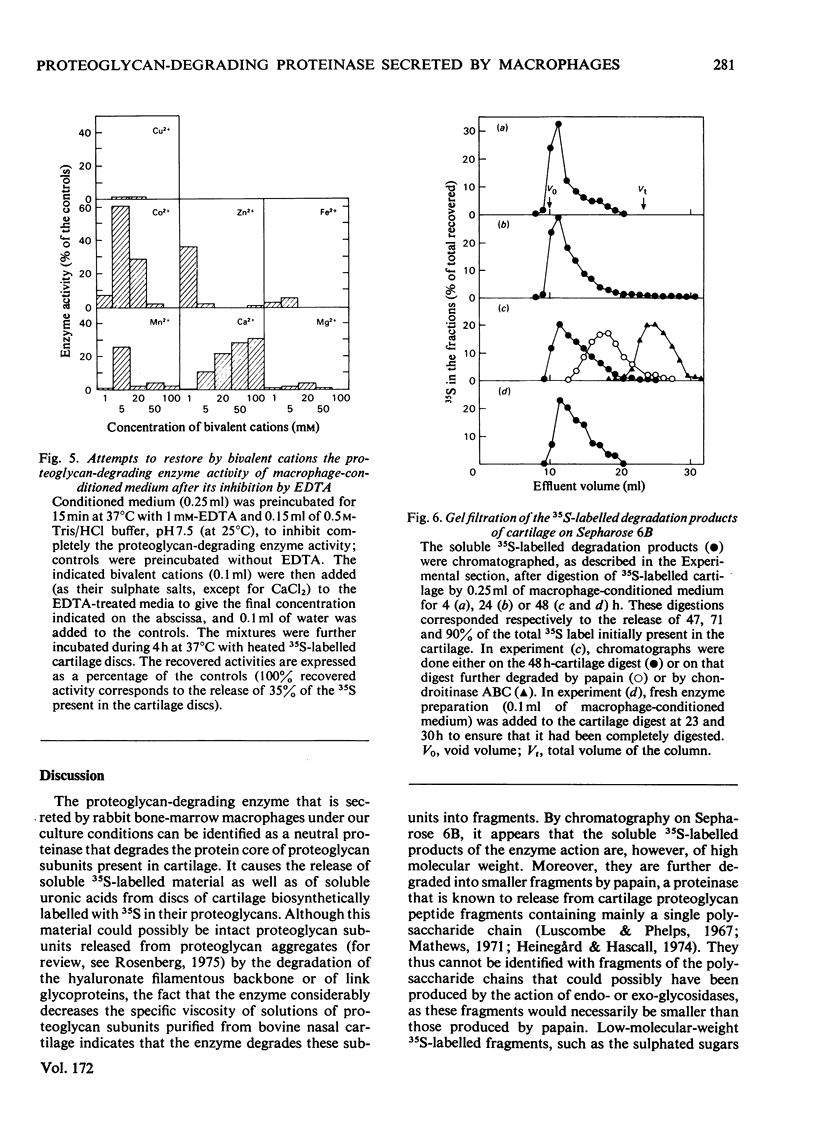
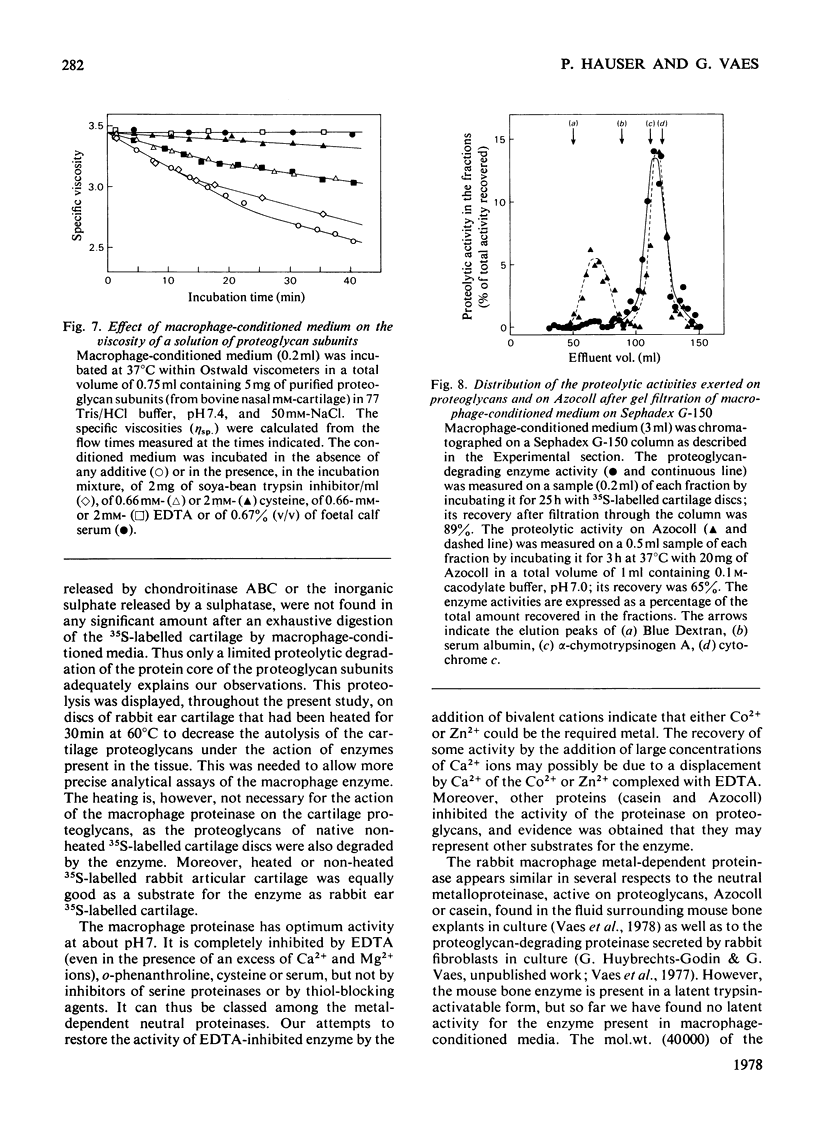


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Ackerman J., Butterfield A., Williams S. Asbestos induces selective release of lysosomal enzymes from mononuclear phagocytes. Nature. 1974 Oct 4;251(5474):423–425. doi: 10.1038/251423a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Recent insights into the pathogenesis of the proliferative lesion in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):68–72. doi: 10.1002/art.1780190111. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hauser P., Vaes G. Synthesis and secretion by rabbit bone-marrow macrophages in culture of a neutral proteinase that degrades cartilage proteoglycans [proceedings]. Biochem Soc Trans. 1977;5(4):1091–1093. doi: 10.1042/bst0051091. [DOI] [PubMed] [Google Scholar]
- Hauser P., Vaes G. The isolation and cultivation of rabbit bone marrow mononuclear phagocytes. Exp Cell Res. 1978 Feb;111(2):353–361. doi: 10.1016/0014-4827(78)90180-5. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- Klimetzek V., Sorg C. Lymphokine-induced secretion of plasminogen activator by murine macrophages. Eur J Immunol. 1977 Mar;7(3):185–187. doi: 10.1002/eji.1830070314. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Krane S. M. IV. Joint erosion in rheumatoid arthritis. Arthritis Rheum. 1974 May-Jun;17(3):306–312. doi: 10.1002/art.1780170316. [DOI] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. Action of degradative enzymes on the light fraction of bovine septa protein polysaccharide. Biochem J. 1967 Apr;103(1):103–109. doi: 10.1042/bj1030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Effects of dental plaque on the production and release of lysosomal hydrolases by macrophages in culture. Arch Oral Biol. 1973 Dec;18(12):1481–1495. doi: 10.1016/0003-9969(73)90124-6. [DOI] [PubMed] [Google Scholar]
- Pantalone R. M., Page R. C. Lymphokine-induced production and release of lysosomal enzymes by macrophages. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2091–2094. doi: 10.1073/pnas.72.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Allison A. C. Effects of activated complement components on enzyme secretion by macrophages. Immunology. 1976 Nov;31(5):781–788. [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Edwards J. H., Davies P., Allison A. C. Macrophage responses to mouldy hay dust, Micropolyspora faeni and zymosan, activators of complement by the alternative pathway. Clin Exp Immunol. 1977 Feb;27(2):198–207. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Eeckhout Y., Lenaers-Claeys G., François-Gillet C., Druetz J. E. The simultaneous release by bone explants in culture and the parallel activation of procollagenase and of a latent neutral proteinase that degrades cartilage proteoglycans and denatured collagen. Biochem J. 1978 May 15;172(2):261–274. doi: 10.1042/bj1720261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Jacques P. Studies on bone enzymes. The assay of acid hydrolases and other enzymes in bone tissue. Biochem J. 1965 Nov;97(2):380–388. doi: 10.1042/bj0970380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]



