Abstract
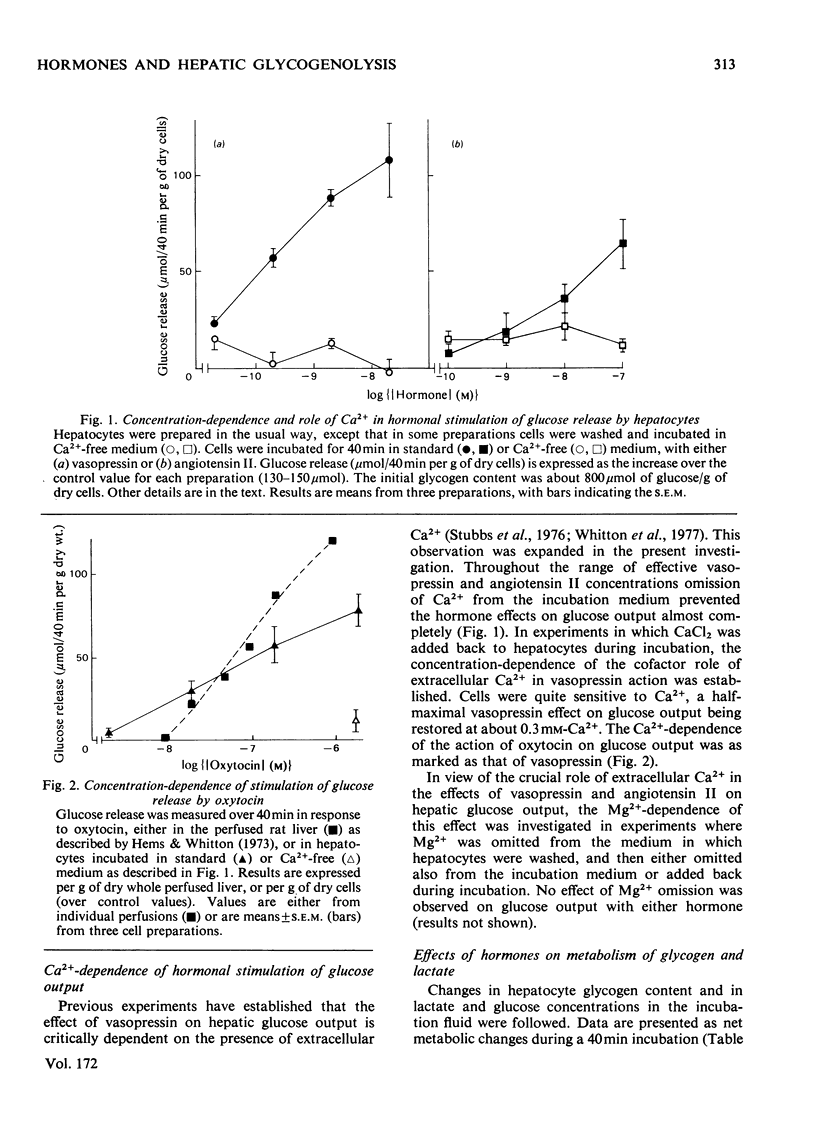
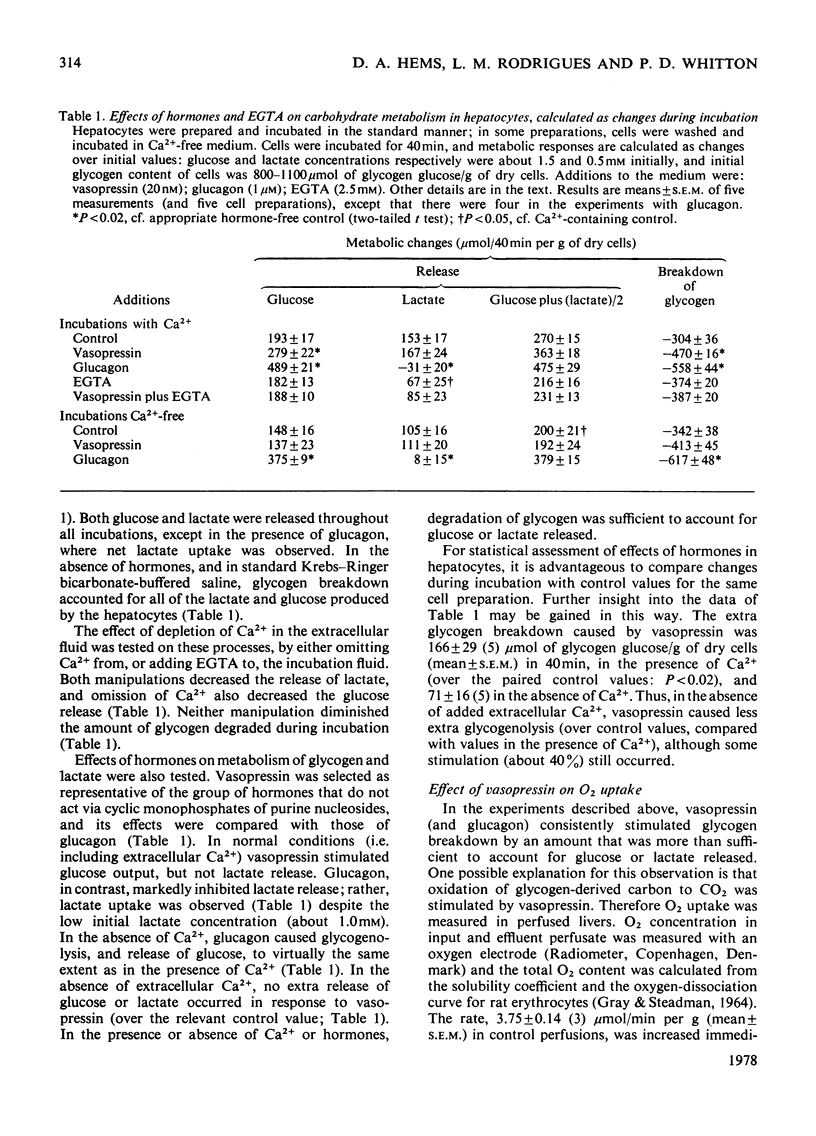
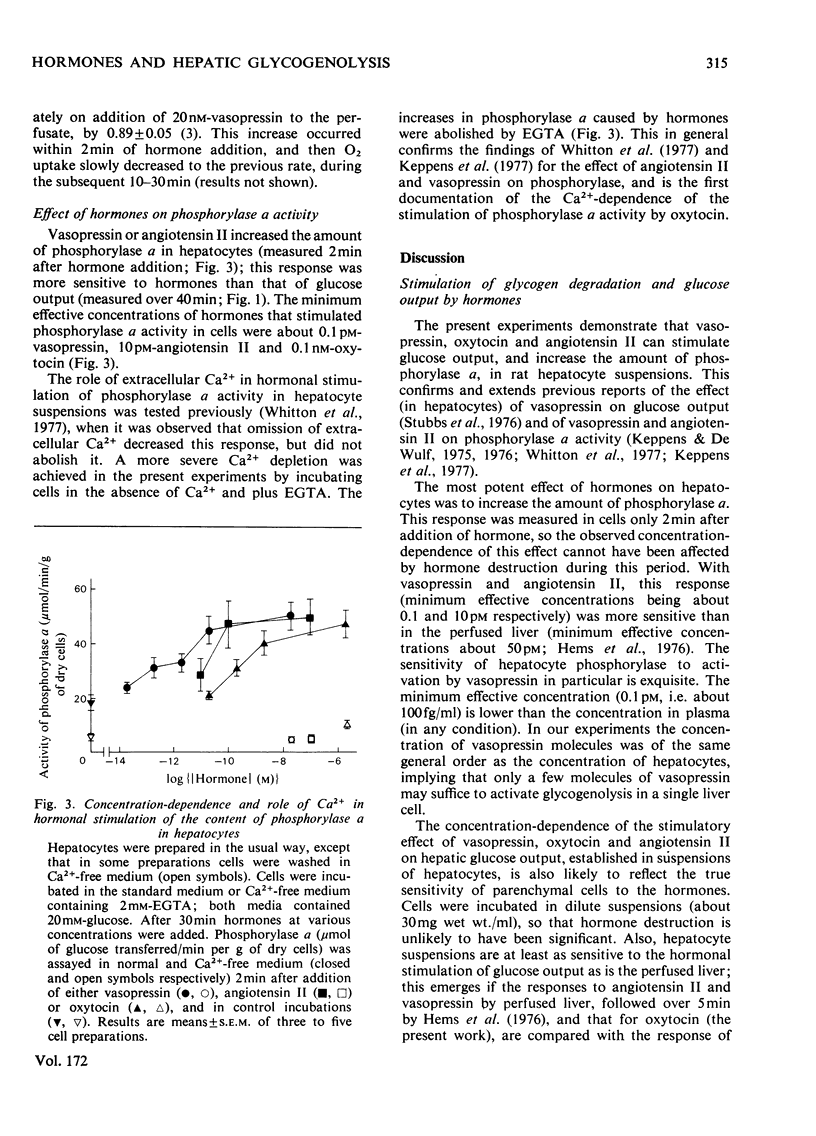
1. The hormonal control of glycogen breakdown was studied in hepatocytes isolated from livers of fed rats. 2. Glucose release was stimulated by [8-arginine]vasopressin (10pm–10nm), oxytocin (1nm–1μm), and angiotensin II (1nm–0.1μm). These responses are all at least as sensitive to hormone as is glucose output in the perfused rat liver. 3. The effect of these three hormones on glucose release was critically dependent on extracellular Ca2+, unlike that of glucagon. Half-maximal restoration of the vasopressin response occurred if 0.3mm-Ca2+ was added back to the incubation medium. 4. Glycogen breakdown was more than sufficient to account for the glucose released into the medium, in the absence or presence of hormones. Lactate release by hepatocytes was not affected by vasopressin, but was inhibited by glucagon. 5. If Ca2+ was omitted from the extracellular medium, vasopressin stimulated glycogenolysis, but not glucose release. 6. The phosphorylase a content of hepatocytes was increased by vasopressin, oxytocin and angiotensin II; minimum effective concentrations were 0.1pm, 0.1nm and 10pm respectively. This response was also dependent on Ca2+. 7. These results demonstrate that hepatocytes can respond to low concentrations of vasopressin and angiotensin II, i.e. these effects are likely to be relevant in the intact animal. The role of extracellular Ca2+ in the effects of these hormones on hepatic glycogenolysis and glucose release is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R. The effects of gelatin and bovine serum albumin on Ca2+ stimulation of gluconeogenesis in isolalted rat hepatocytes. FEBS Lett. 1976 Apr 15;64(1):62–64. doi: 10.1016/0014-5793(76)80249-9. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., STEADMAN J. M. DETERMINATION OF THE OXYHAEMOGLOBIN DISSOCIATION CURVES FOR MOUSE AND RAT BLOOD. J Physiol. 1964 Dec;175:161–171. doi: 10.1113/jphysiol.1964.sp007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Glycogen phosphorylase, glucose output and vasoconstriction in the perfused rat liver. Concentration-dependence of actions of adrenaline, vasopressin and angiotensin II. Biochem J. 1976 Nov 15;160(2):367–374. doi: 10.1042/bj1600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Ma G. Y. Metabolic actions of vasopressin, glucagon and adrenalin in the intact rat. Biochim Biophys Acta. 1975 Nov 10;411(1):155–164. doi: 10.1016/0304-4165(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. The activation of liver glycogen phosphorylase by angiotensin II. FEBS Lett. 1976 Oct 1;68(2):279–282. doi: 10.1016/0014-5793(76)80453-x. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Keppens S., de Wulf H. The activation of liver glycogen phosphorylase by vasopressin. FEBS Lett. 1975 Mar 1;51(1):29–32. doi: 10.1016/0014-5793(75)80848-9. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Steinberg D. Stimulation of rat liver phosphorylase kinase by micromolar concentrations of Ca2+. FEBS Lett. 1975 Sep 1;57(1):68–72. doi: 10.1016/0014-5793(75)80154-2. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Hems D. A. Hepatic action of vasopressin: lack of a role for adenosine-3',5'-cyclic monophosphate. FEBS Lett. 1974 Oct 1;47(1):128–131. doi: 10.1016/0014-5793(74)80441-2. [DOI] [PubMed] [Google Scholar]
- Ma G. Y., Hems D. A. Inhibition of fatty acid synthesis and stimulation of glycogen breakdown by vasopressin in the perfused mouse liver. Biochem J. 1975 Nov;152(2):389–392. doi: 10.1042/bj1520389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Kirk C. J., Hems D. A. Role of extracellular calcium in the action of vasopressin on hepatic glycogenolysis. FEBS Lett. 1976 Oct 15;69(1):199–202. doi: 10.1016/0014-5793(76)80686-2. [DOI] [PubMed] [Google Scholar]
- Whitton P. D., Hems D. A. Actions of vasopressin-related peptides on glycogen metabolism in the perfused rat liver. Biochem Pharmacol. 1976 Feb 15;25(4):405–407. doi: 10.1016/0006-2952(76)90341-5. [DOI] [PubMed] [Google Scholar]
- Whitton P. D., Rodrigues L. M., Hems D. A. Influence of extracellular calcium ions on hormonal stimulation of glycogen breakdown in hepatocyte suspensions [proceedings]. Biochem Soc Trans. 1977;5(4):992–994. doi: 10.1042/bst0050992. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Hue L., Hers H. G. Hormonal and ionic control of the glycogenolytic cascade in rat liver. Biochem J. 1977 Jan 15;162(1):135–142. doi: 10.1042/bj1620135. [DOI] [PMC free article] [PubMed] [Google Scholar]



