Abstract
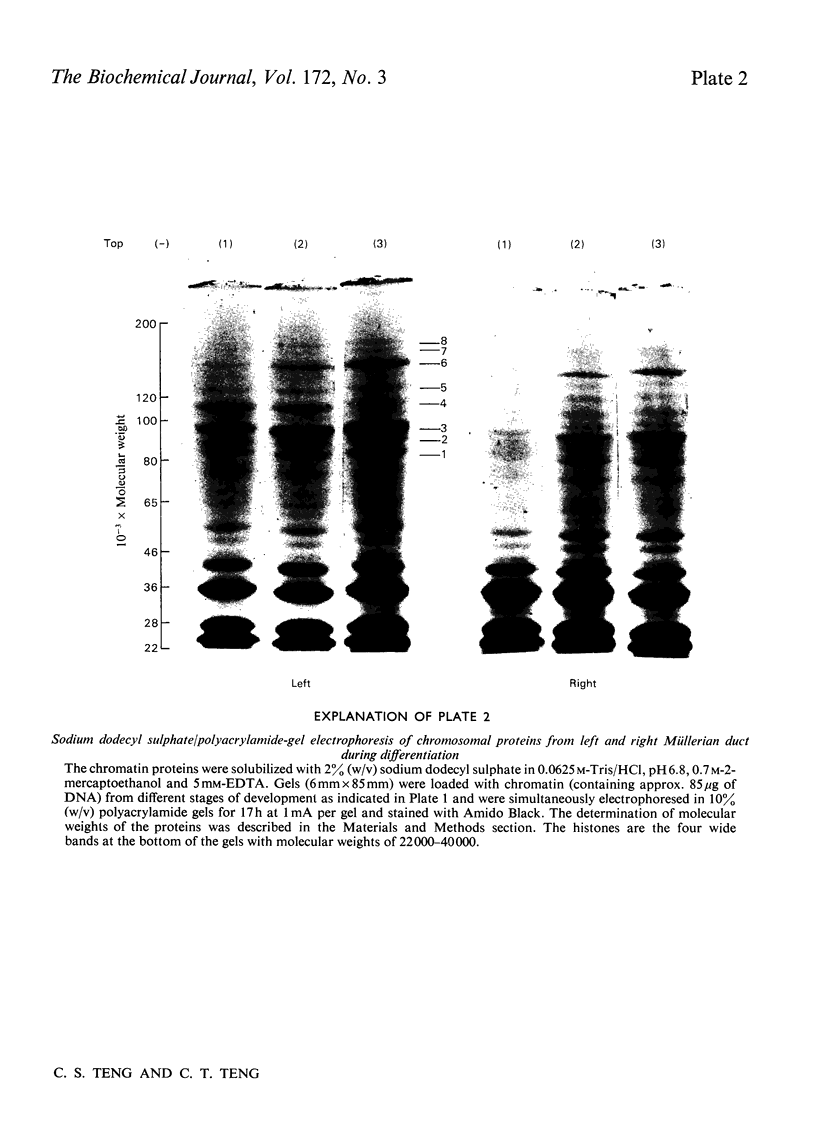
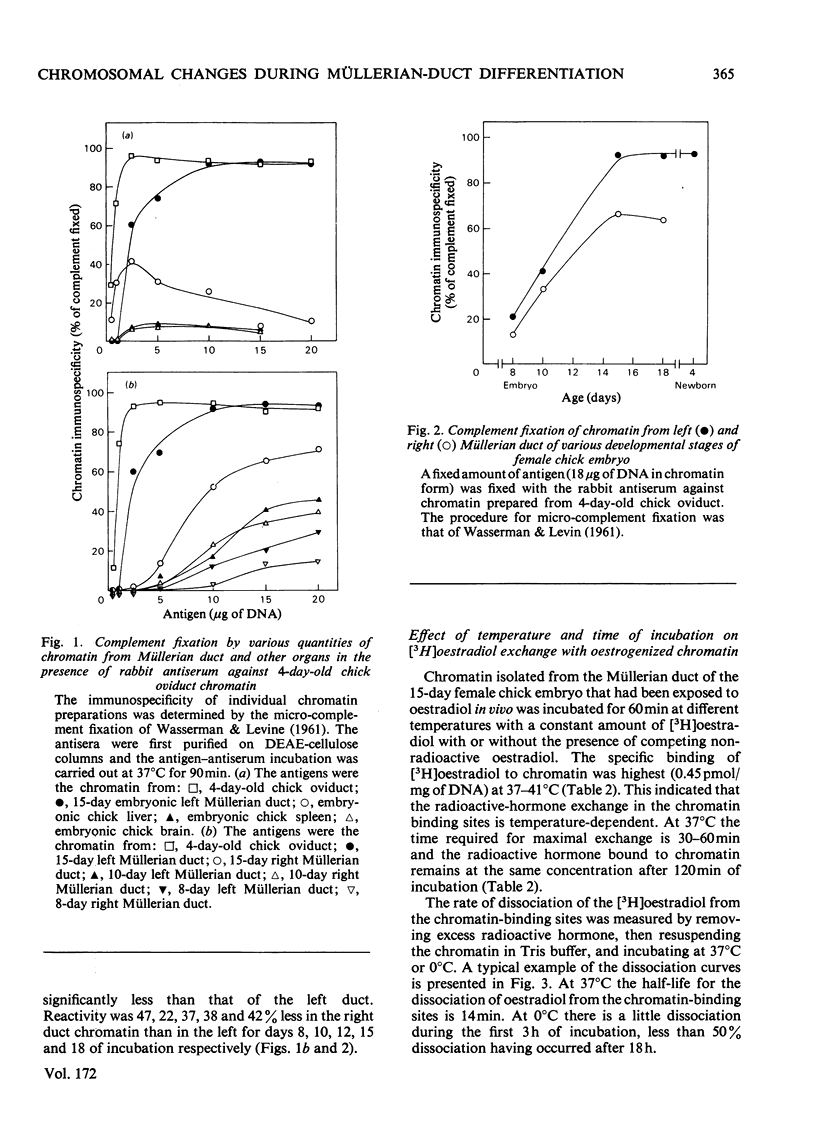
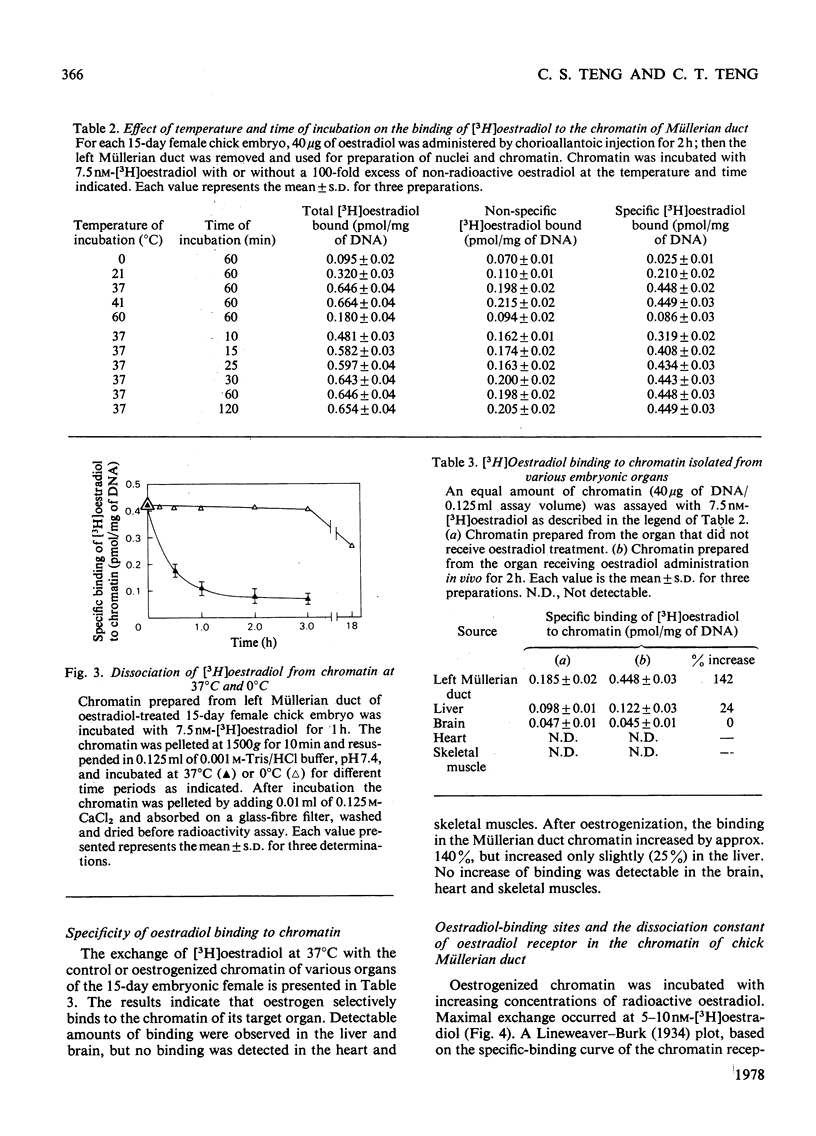
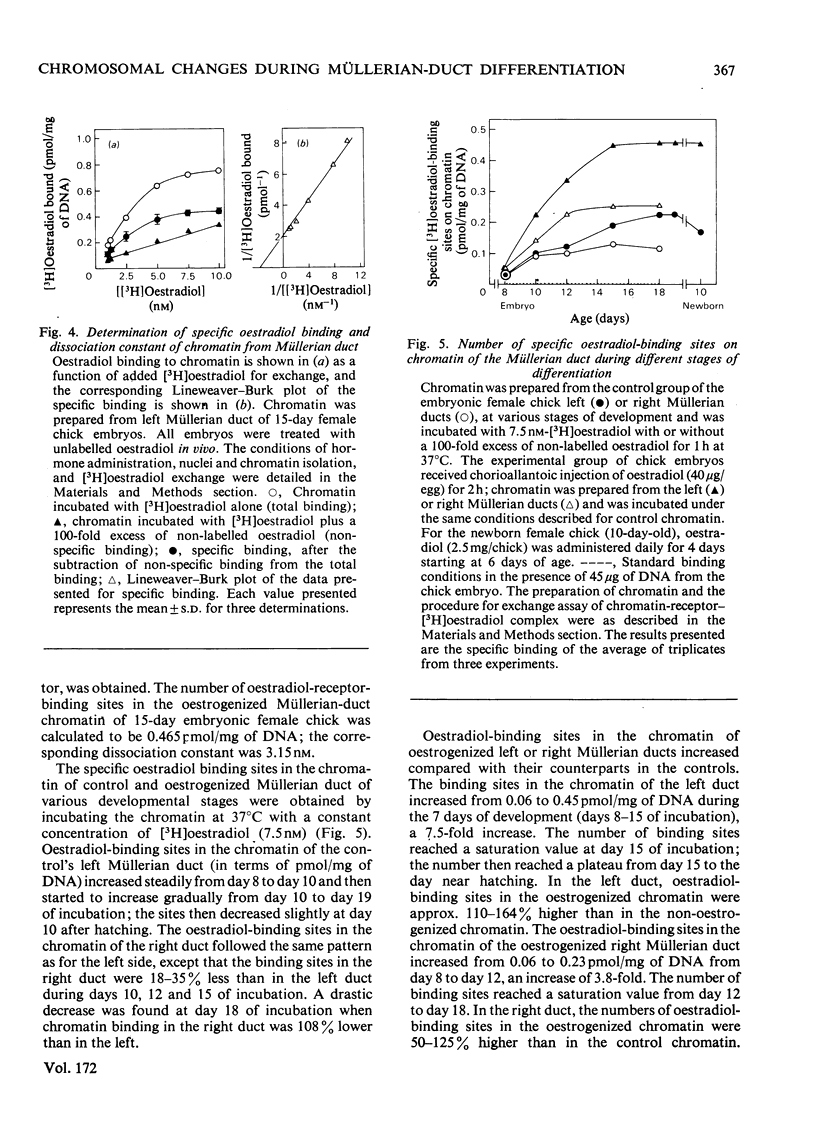
Biochemical and immunochemical techniques were used to probe the changes in composition of the chromatin of differentiating Müllerian ducts. The non-histone protein increases gradually in the left duct and reaches a constant amount at day 15 of incubation, then remains at the same value until after birth. In the regressing right duct, the non-histone protein increases and then decreases. Gel electrophoresis indicated an increased heterogeneity in the composition of the non-histone protein corresponding to Müllerian-duct differentiation. Little variation in quantity and quality of the histone was observed; however, immunochemical assay confirmed the structural change of Müllerian-duct chromatin during development. An antibody against the chromatin of the newborn-chick oviduct was produced in the rabbit. The chromatin of Müllerian ducts from the early embryonic stage showed a small affinity with the antibody; the affinity increased during the late embryonic stages. The affinity was greatly decreased in the regressing right duct. Oestrogen-binding sites were present in the chromatin of the left and right Müllerian ducts during differentiation, with more sites in the left duct than in the right one during the late stages of development. After oestrogen treatment in vivo, the oestrogen-binding sites on the chromatin of both the left and the right ducts were increased, with a greater increase in the left duct than in the right. In the developing left duct the binding sites reach a maximum on day 15 of incubation, and remain constant at that value until birth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. E., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. IX. The kinetics of nuclear binding. J Biol Chem. 1975 Feb 10;250(3):809–818. [PubMed] [Google Scholar]
- Chamness G. C., Jennings A. W., McGuire W. L. Estrogen receptor binding to isolated nuclei. A nonsaturable process. Biochemistry. 1974 Jan 15;13(2):327–331. doi: 10.1021/bi00699a016. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Hunt M., Hnilica L. S. Tissue-specific DNA-protein complexes during azo dye hepatocarcinogenesis. Cancer Res. 1975 Apr;35(4):913–919. [PubMed] [Google Scholar]
- Chytil F., Glasser S. R., Splesberg T. C. Alterations in liver chromatin during perinatal development of the rat. Dev Biol. 1974 Apr;37(2):295–305. doi: 10.1016/0012-1606(74)90150-x. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Cohen M. E., Hamilton T. H. Effect of extradiol-17beta on the synthesis of specific uterine nonhistone chromosomal proteins. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4346–4350. doi: 10.1073/pnas.72.11.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hertogh R., Ekka E., Vanderheyden I., Hoet J. J. Slowly exchangeable pool of estradiol in the rat uterus. J Steroid Biochem. 1973 May;4(3):313–320. doi: 10.1016/0022-4731(73)90055-1. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. Solubilization of the chromatin-bound estrogen receptor from chicken liver and fractionation on hydroxylapatite. Eur J Biochem. 1976 Aug 16;67(2):411–419. doi: 10.1111/j.1432-1033.1976.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Binding of the estradiol receptor to hen oviduct nuclei and chromatin. J Steroid Biochem. 1976 May;7(5):413–418. doi: 10.1016/0022-4731(76)90104-7. [DOI] [PubMed] [Google Scholar]
- Jaffe R. C., Socher S. H., O'Malley B. W. An analysis of the binding of the chick oviduct progesterone-receptor to chromatin. Biochim Biophys Acta. 1975 Aug 13;399(2):403–419. doi: 10.1016/0304-4165(75)90269-x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Desombre E. R., Hurst D. J., Kawashima T., Jungblut P. W. Estrogen-receptor interactions in target tissues. Arch Anat Microsc Morphol Exp. 1967;56(3):547–569. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Baulieu E. E. An insoluble receptor for oestrogens in the "residual" nuclear proteins of chick-liver. Eur J Biochem. 1973 Jul 2;36(1):294–300. doi: 10.1111/j.1432-1033.1973.tb02913.x. [DOI] [PubMed] [Google Scholar]
- Loeb J. E., Creuzet C. Comparaison des propriétés électrophorétiques des protéines nucléaires de différents tissus. Bull Soc Chim Biol (Paris) 1970;52(10):1007–1020. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Peterken B. M. A reconstituted cell-free system for the specific transfer of steroid--receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J. 1971 Nov;125(1):285–295. doi: 10.1042/bj1250285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraud R., Stoll R., Coulaud H. Données nouvelles sur le role du testicule et de l'hypophyse dans la différenciation sexuelle du poulet. C R Assoc Anat. 1970 Sep;148:442–449. [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Ozaki H. Properties of isolated chromatin from sea urchin embryo. Dev Biol. 1967 Nov;16(5):474–488. doi: 10.1016/0012-1606(67)90060-7. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Dynamics of oestrogen-receptor distribution between the cytosol and nuclear fractions of immature rat uterus after oestradiol administration. Biochem J. 1975 Mar;146(3):617–623. doi: 10.1042/bj1460617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in nuclear and chromatin composition and genomic activity during spermatogenesis in the maturing rooster testis. Biochem J. 1977 Apr 15;164(1):99–111. doi: 10.1042/bj1640099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Nyberg L. M., Wang T. Y. The role of the androgen-binding nonhistone proteins in the transcription of prostatic chromatin. J Steroid Biochem. 1976 Apr;7(4):267–273. doi: 10.1016/0022-4731(76)90126-6. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Platz R. D., Grimes S. R., Meistrich M. L., Hnilica L. S. Changes in nuclear proteins of rat testis cells separated by velocity sedimentation. J Biol Chem. 1975 Aug 10;250(15):5791–5800. [PubMed] [Google Scholar]
- Puca G. A., Sica V., Nola E. Identification of a high affinity nuclear acceptor site for estrogen receptor of calf uterus. Proc Natl Acad Sci U S A. 1974 Mar;71(3):979–983. doi: 10.1073/pnas.71.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- SCHEIB-PFLEGER D., WATTIAUX R. Study of the acid hydrolases of the muellerian ducts of the chick embryo. I. Total activity and solvent activity of the ducts of embryos after 8 to 10 days of incubation. Dev Biol. 1962 Oct;5:205–217. doi: 10.1016/0012-1606(62)90010-6. [DOI] [PubMed] [Google Scholar]
- Seale R. L., Aronson A. I. Chromatin-associated proteins of the developing sea urchin embryo. I. Kinetics of synthesis and characterization of non-histone proteins. J Mol Biol. 1973 Apr 25;75(4):633–645. doi: 10.1016/0022-2836(73)90297-0. [DOI] [PubMed] [Google Scholar]
- Shelton K. R., Allfrey V. G. Selective synthesis of a nuclear acidic protein in liver cells stimulated by cortisol. Nature. 1970 Oct 10;228(5267):132–134. doi: 10.1038/228132a0. [DOI] [PubMed] [Google Scholar]
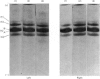
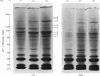
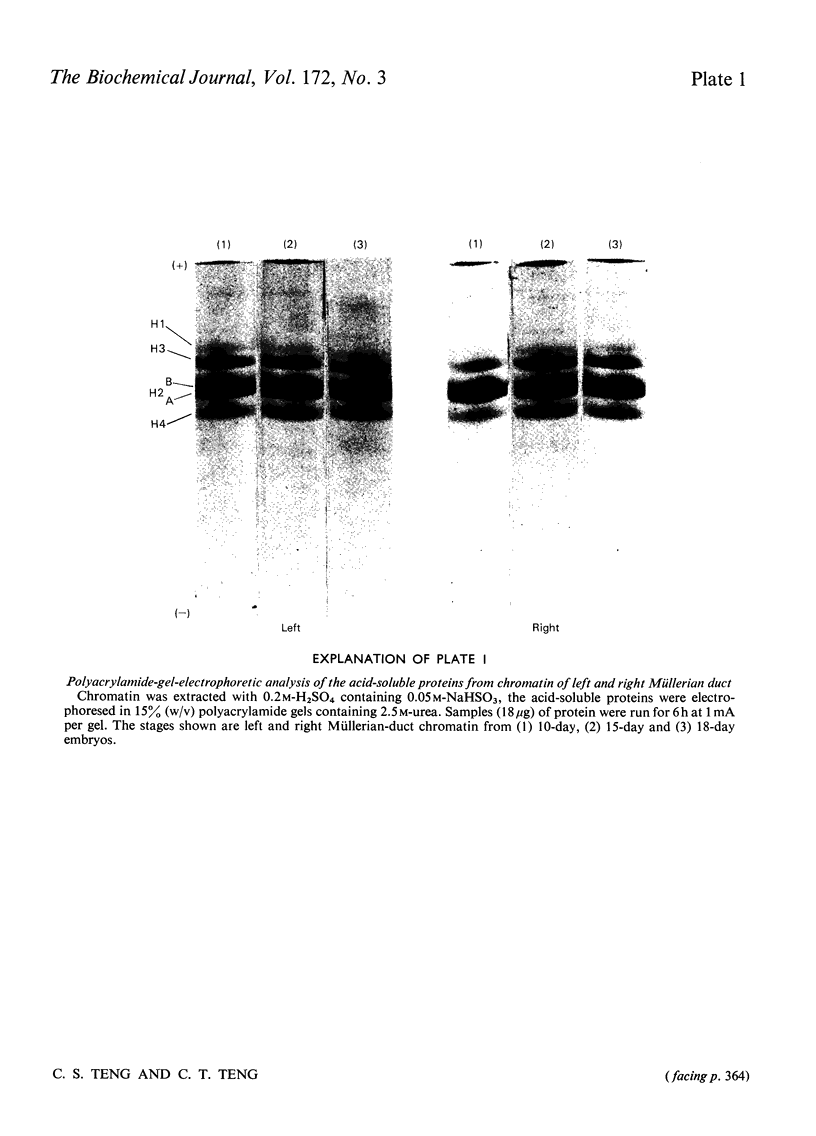
- Spelsberg T. C., Mitchell W. M., Chytil F., Wilson E. M., O'Malley B. W. Chromatin of the developing chick oviduct: changes in the acidic proteins. Biochim Biophys Acta. 1973 Jul 27;312(4):765–778. doi: 10.1016/0005-2787(73)90080-4. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Webster R. A., Pikler G. M. Chromosomal proteins regulate steroid binding to chromatin. Nature. 1976 Jul 1;262(5563):65–67. doi: 10.1038/262065a0. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Spelsberg T. C., Glasser S. R., O'Malley B. W. Soluble complexes between steroid hormones and target-tissue receptors bind specifically to target-tissue chromatin. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1479–1482. doi: 10.1073/pnas.68.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Regulation by estrogen of organ-specific synthesis of a nuclear acidic protein. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1231–1238. doi: 10.1016/0006-291x(70)90927-7. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Role of chromatin in estrogen action in the uterus. II. Hormone-induced synthesis of nonhistone acidic proteins which restore histone-inhibited DNA-dependent RNA synthesis. Proc Natl Acad Sci U S A. 1969 Jun;63(2):465–472. doi: 10.1073/pnas.63.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. The role of chromatin in estrogen action in the uterus, I. The control of template capacity and chemical composition and the binding of H3-estradiol-17 beta. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1410–1417. doi: 10.1073/pnas.60.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S. Nuclear acidic protein of the developing Oncopeltus embryos. Biochim Biophys Acta. 1974 Nov 6;366(4):385–395. doi: 10.1016/0005-2787(74)90036-7. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Isolation and characterization of an oestrogen receptor from chick Müllerian duct. Biochem J. 1975 Aug;150(2):183–190. doi: 10.1042/bj1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Ontogeny of cytoplasmic oestrogen receptor in chick Müllerian duct. Biochem J. 1975 Aug;150(2):191–194. doi: 10.1042/bj1500191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Study on sex-organ development. Oestrogen-receptor translocation in the developing chick Müllerian duct. Biochem J. 1976 Jan 15;154(1):1–9. doi: 10.1042/bj1540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. T., Teng C. S. Studies on sex-organ development. The hormonal regulation of steroidogenesis and adenosine 3':5'-cyclic monophosphate in embryonic-chick ovary. Biochem J. 1977 Jan 15;162(1):123–134. doi: 10.1042/bj1620123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault J., Landesman R. An analysis of acidic nuclear proteins during the development of Xenopus laevis. Cell Differ. 1974 Dec;3(5):249–257. doi: 10.1016/0045-6039(74)90015-3. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Littau V. C., Allfrey K. M., Allfrey V. G. Changes in nuclear acidic protein complement of red blood cells during embryonic development. J Biol Chem. 1973 Jun 10;248(11):4065–4068. [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem. 1974 Nov 25;249(22):7076–7086. [PubMed] [Google Scholar]
- de Boer W., de Vries J., Mulder E., van der Molen H. J. Comparative study of nuclear binding sites for oestradiol in rat testicular and uterine tissue. Determination of low amounts of specific binding site by an [3H] oestradiol-exchange method. Biochem J. 1977 Feb 15;162(2):331–339. doi: 10.1042/bj1620331. [DOI] [PMC free article] [PubMed] [Google Scholar]