Abstract
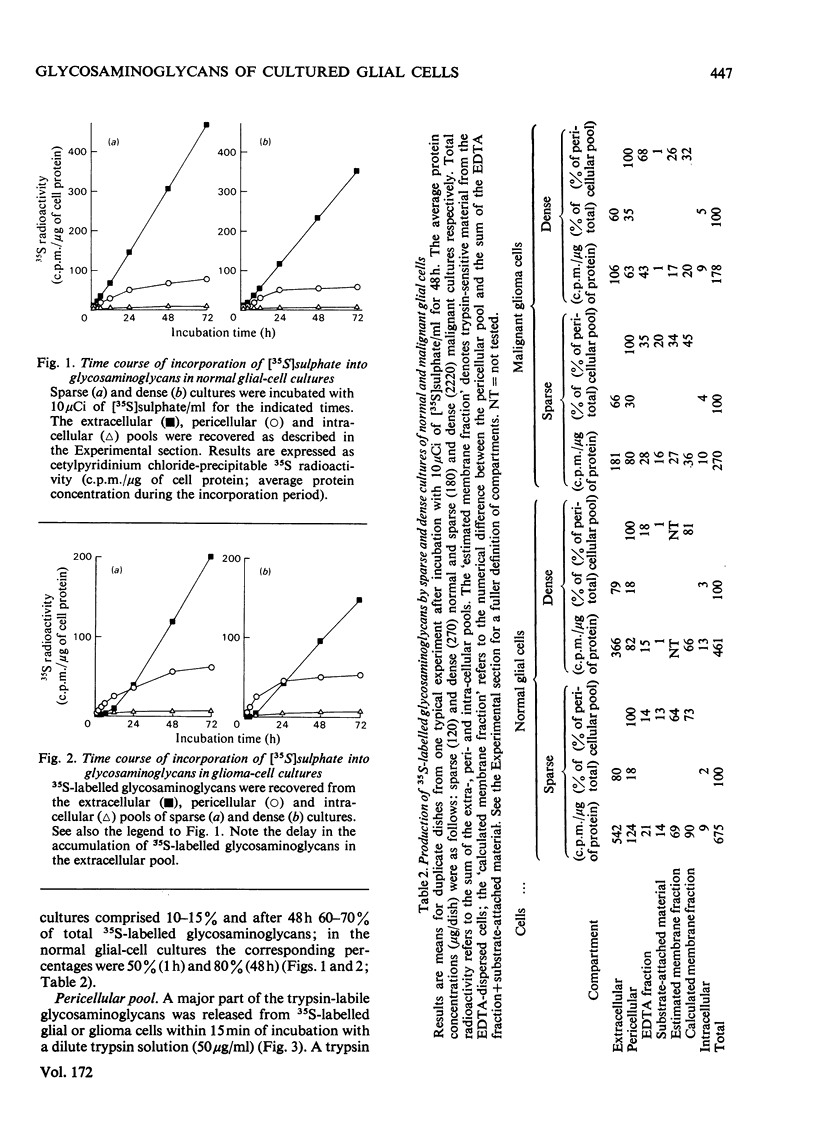
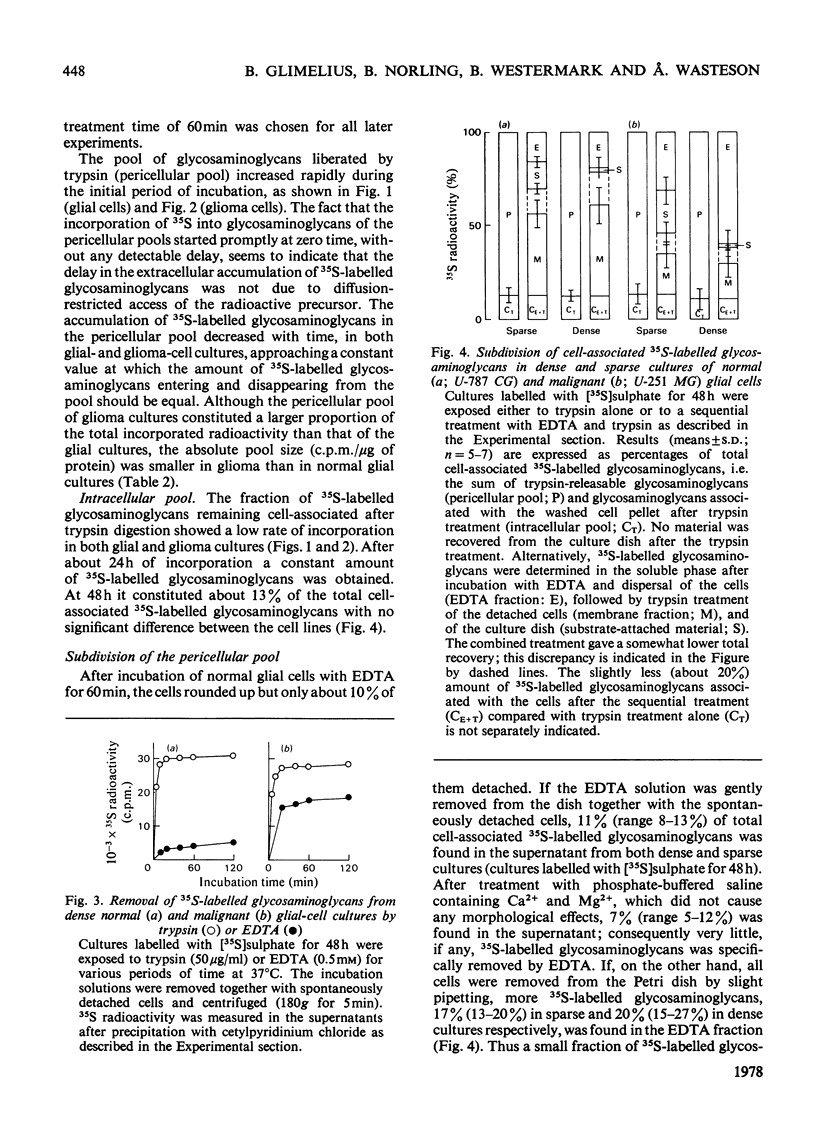
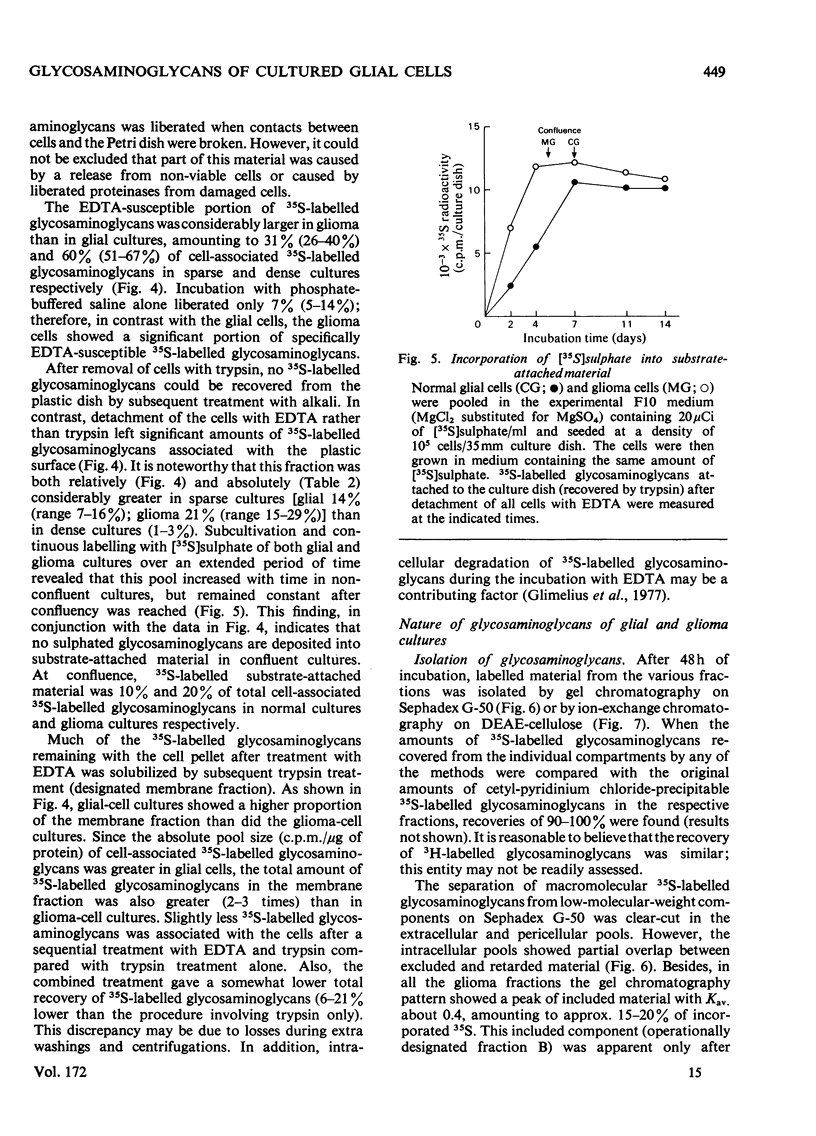
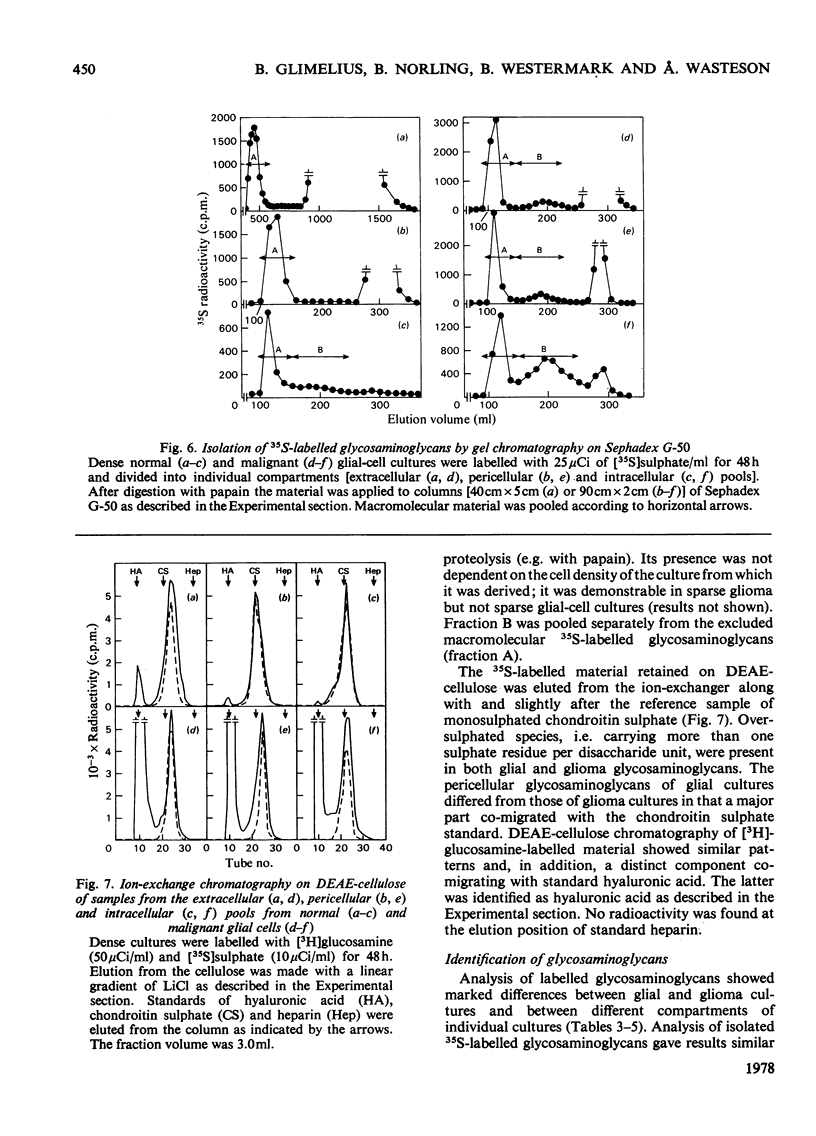
The glycosaminoglycans of human cultured normal glial and malignant glioma cells were studied. [35S]Sulphate or [3H]glucosamine added to the culture medium was incorporated into glycosaminoglycans; labelled glycosaminoglycans were isolated by DEAE-cellulose chromatography or gel chromatography. A simple procedure was developed for measurement of individual sulphated glycosaminoglycans in cell-culture fluids. In normal cultures the glycosaminoglycans of the pericellular pool (trypsin-susceptible material), the membrane fraction (trypsin-susceptible material of EDTA-detached cells) and the substrate-attached material consisted mainly of heparan sulphate. The intra- and extra-cellular pools showed a predominance of dermatan sulphate. The net production of hyaluronic acid was low. The accumulation of 35S-labelled glycosaminoglycans in the extracellular pool was essentially linear with time up to 72h. The malignant glioma cells differed in most aspects tested. The total production of glycosaminoglycans was much greater owing to a high production of hyaluronic acid and hyaluronic acid was the major cell-surface-associated glycosaminoglycan in these cultures. Among the sulphated glycosaminoglycans chondroitin sulphate, rather than heparan sulphate, was the predominant species of the pericellular pool. This was also true for the membrane fraction and substrate-attached material. Furthermore, the accumulation of extracellular 35S-labelled glycosaminoglycans was initially delayed for several hours and did not become linear with time until after 24 h of incubation. The glioma cells produced little dermatan sulphate and the dermatan sulphate chains differed from those of normal cultures with respect to the distribution of iduronic acid residues. The observed differences between normal glial and malignant glioma cells were not dependent on cell density; rather they were due to the malignant transformation itself.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadò R., Ingmar B., Lindahl U., Wasteson A. Depolymerisation and desulphation of chondroitin sulphate by enzymes from embryonic chick cartilage. FEBS Lett. 1974 Feb 1;39(1):49–52. doi: 10.1016/0014-5793(74)80014-1. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Cohn R. H., Cassiman J. J., Bernfield M. R. Relationship of transformation, cell density, and growth control to the cellular distribution of newly synthesized glycosaminoglycan. J Cell Biol. 1976 Oct;71(1):280–294. doi: 10.1083/jcb.71.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Release of macromolecules from BALB-c mouse cell lines treated with chelating agents. Biochemistry. 1972 May 23;11(11):2161–2172. doi: 10.1021/bi00761a024. [DOI] [PubMed] [Google Scholar]
- Culp L. A. Molecular composition and origin of substrate-attached material from normal and virus-transformed cells. J Supramol Struct. 1976;5(2):239–255. doi: 10.1002/jss.400050210. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Terry A. H., Buniel J. F. Metabolic properties of substrate-attached glycoproteins from normal and virus-transformed cells. Biochemistry. 1975 Jan 28;14(2):406–412. doi: 10.1021/bi00673a030. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Ho P. L. Synthesis of acid mucopolysaccharides by glial tumor cells in tissue culture. Proc Natl Acad Sci U S A. 1970 Jun;66(2):495–499. doi: 10.1073/pnas.66.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Davis M. L., Trammell D. J., Stevens R. L., Kihara H. Mucopolysaccharides synthesized by cultured glial cells derived from a patient with Sanfilippo A syndrome. J Neurochem. 1975 Oct;25(4):429–435. doi: 10.1111/j.1471-4159.1975.tb04345.x. [DOI] [PubMed] [Google Scholar]
- Glimelius B., Westermark B., Wasteson A. Ammonium ion interferes with the lysosomal degradation of glycosaminoglycans in cultures of human glial cells. Exp Cell Res. 1977 Aug;108(1):23–30. doi: 10.1016/s0014-4827(77)80005-0. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Todaro G. J., Green H. The production of hyaluronate by spontaneously established cell lines and viral transformed lines of fibroblastic origin. Biochim Biophys Acta. 1965 Nov 1;101(3):343–351. doi: 10.1016/0926-6534(65)90013-8. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Hallén A., Bäckström G. Biosynthesis of heparin. Studies on the microsomal sulfation process. J Biol Chem. 1975 Aug 10;250(15):6065–6071. [PubMed] [Google Scholar]
- Ishimoto N., Temin H. M., Strominger J. L. Studies of carcinogenesis by avian sarcoma viruses. II. Virus-induced increase in hyaluronic acid synthetase in chicken fibroblasts. J Biol Chem. 1966 May 10;241(9):2052–2057. [PubMed] [Google Scholar]
- Kleinman H. K., Silbert J. E., Silbert C. K. Heparan sulfate of skin fibroblasts grown in culture. Connect Tissue Res. 1975;4(1):17–23. doi: 10.3109/03008207509152193. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. II. Acid-soluble and -precipitable species of different cell lines. Biochemistry. 1971 Apr 13;10(8):1445–1451. doi: 10.1021/bi00784a027. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lohmander S. Ion exchange chromatography of glucosamine and galactosamine in microgram amounts with quantitative determination and specific radioactivity assay. Biochim Biophys Acta. 1972 May 16;264(3):411–417. doi: 10.1016/0304-4165(72)90003-7. [DOI] [PubMed] [Google Scholar]
- Malström A., Carlstedt I., Aberg L., Fransson L. A. The copolymeric structure of dermatan sulphate produced by cultured human fibroblasts. Different distribution of iduronic acid and glucuronic acid-containing units in soluble and cell-associated glycans. Biochem J. 1975 Dec;151(3):477–489. doi: 10.1042/bj1510477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. U., Atherton D. M. The heparan sulfate of rat brain. Biochim Biophys Acta. 1972 Jul 19;273(2):368–373. doi: 10.1016/0304-4165(72)90228-0. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Margolis R. K. Distribution and metabolism of mucopolysaccharides and glycoproteins in neuronal perikarya, astrocytes, and oligodendroglia. Biochemistry. 1974 Jul 2;13(14):2849–2852. doi: 10.1021/bi00711a011. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Ohshima E., Ohtsuka M. Effect of liver cell coat acid mucopolysaccharide on the appearance of density-dependent inhibition in hepatoma cell growth. Exp Cell Res. 1975 Jun;93(1):136–142. doi: 10.1016/0014-4827(75)90432-2. [DOI] [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Pontén J., Westermark B., Hugosson R. Regulation of proliferation and movement of human glialike cells in culture. Exp Cell Res. 1969 Dec;58(2):393–400. doi: 10.1016/0014-4827(69)90520-5. [DOI] [PubMed] [Google Scholar]
- Roblin R., Albert S. O., Gelb N. A., Black P. H. Cell surface changes correlated with density-dependent growth inhibition. Glycosaminoglycan metabolism in 3T3, SV3T3, and con A selected revertant cells. Biochemistry. 1975 Jan 28;14(2):347–357. doi: 10.1021/bi00673a022. [DOI] [PubMed] [Google Scholar]
- Satoh C., Duff R., Rapp F., Davidson E. A. Production of mucopolysaccharides by normal and transformed cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):54–56. doi: 10.1073/pnas.70.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry A. H., Culp L. A. Substrate-attached glycoproteins from normal and virus-transformed cells. Biochemistry. 1974 Jan 29;13(3):414–425. doi: 10.1021/bi00700a004. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Keller J. M. A transformation-dependent difference in the heparan sulfate associated with the cell surface. Biochem Biophys Res Commun. 1975 Mar 17;63(2):448–454. doi: 10.1016/0006-291x(75)90708-1. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Keller J. M. Density-dependent changes in the amount of sulfated glycosaminoglycans associated with mouse 3T3 cells. J Cell Physiol. 1976 Sep;89(1):53–63. doi: 10.1002/jcp.1040890106. [DOI] [PubMed] [Google Scholar]
- Vannucchi S., Chiarugi V. P. Surface exposure of glycosaminoglycans in resting, growing and virus transformed 3T3 cells. J Cell Physiol. 1977 Mar;90(3):503–509. doi: 10.1002/jcp.1040900313. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Glimelius B., Busch C., Westermark H., Heldin C. H., Norling B. Effect of a platelet endoglycosidase on cell surface associated heparan sulphate of human culturei endothelial and glial cells. Thromb Res. 1977 Sep;11(3):309–321. doi: 10.1016/0049-3848(77)90184-0. [DOI] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A., Westermark B., Lindahl U., Pontén J. Aggregation of feline lymphoma cells by hyaluronic acid. Int J Cancer. 1973 Jul 15;12(1):169–178. doi: 10.1002/ijc.2910120118. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Westermark B., Uthne K. Somatomedin A and B: demonstration of two different somatomedinlike components in human plasma. Adv Metab Disord. 1975;8:101–113. doi: 10.1016/b978-0-12-027308-9.50013-5. [DOI] [PubMed] [Google Scholar]
- Westermark B., Pontén J., Hugosson R. Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand A. 1973 Nov;81(6):791–805. doi: 10.1111/j.1699-0463.1973.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Westermark B. The deficient density-dependent growth control of human malignant glioma cells and virus-transformed glia-like cells in culture. Int J Cancer. 1973 Sep 15;12(2):438–451. doi: 10.1002/ijc.2910120215. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


