Abstract
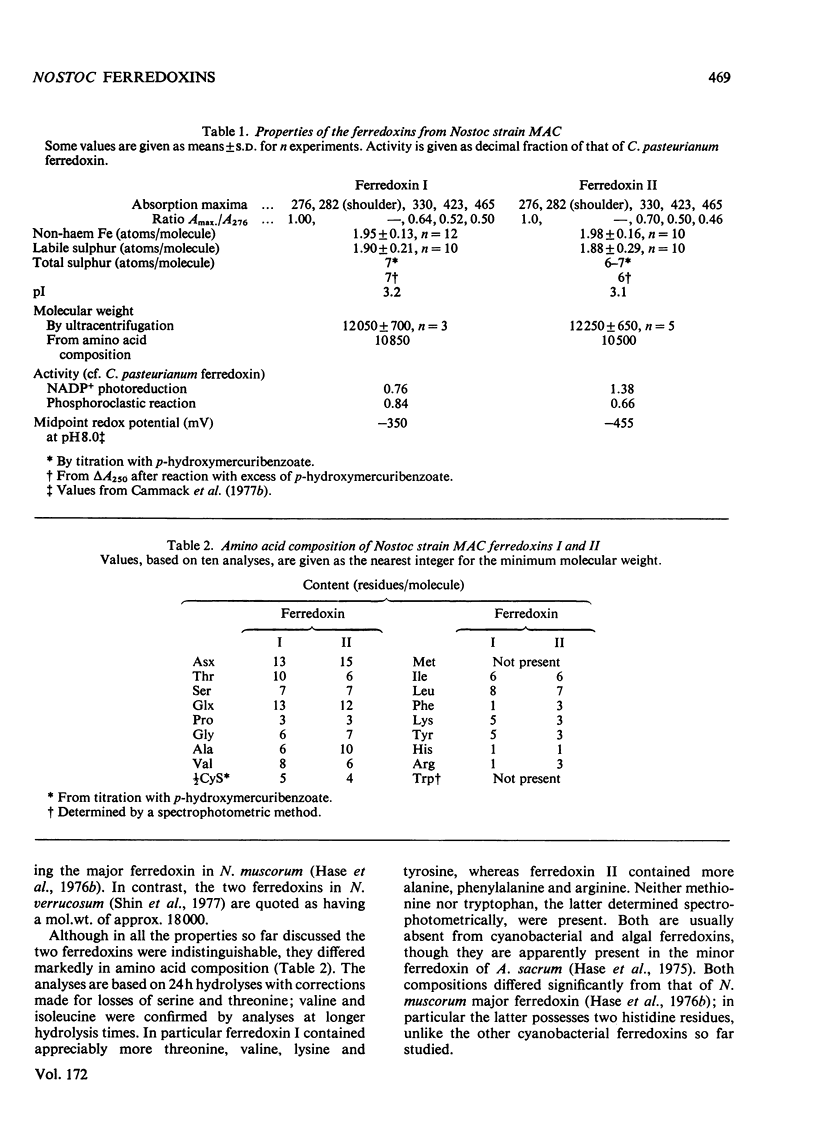
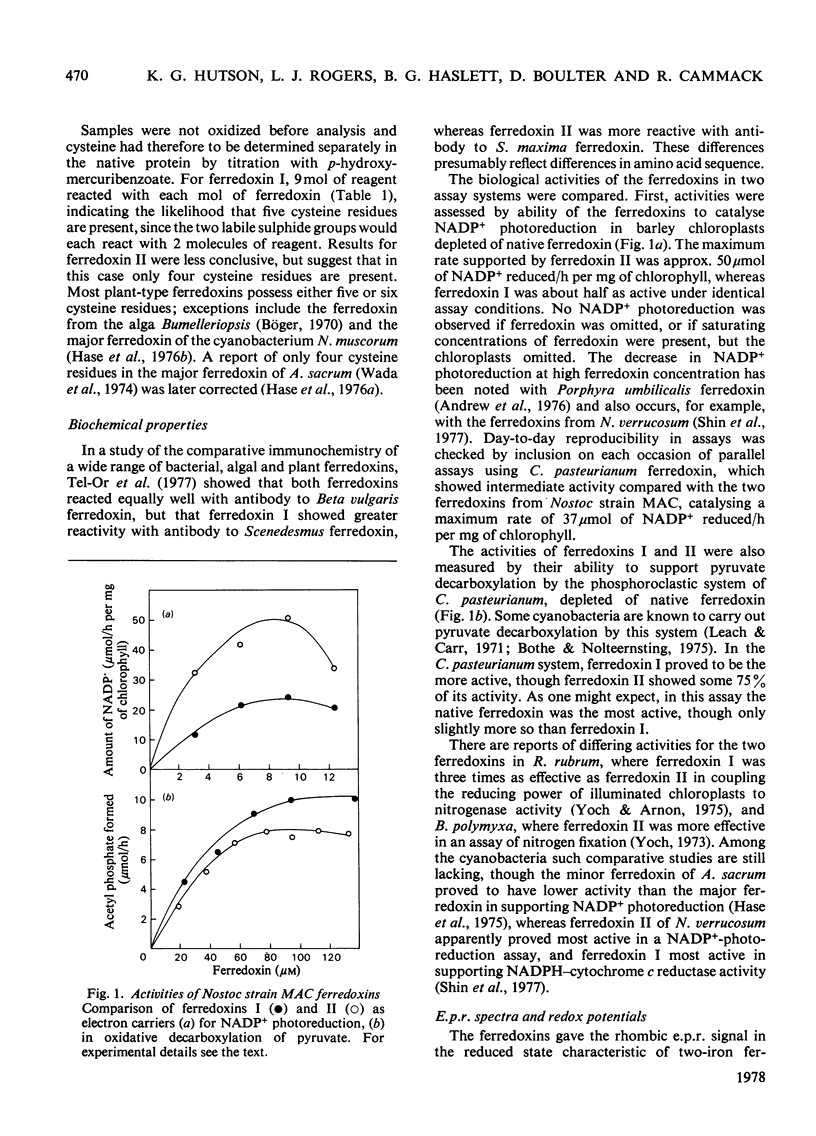
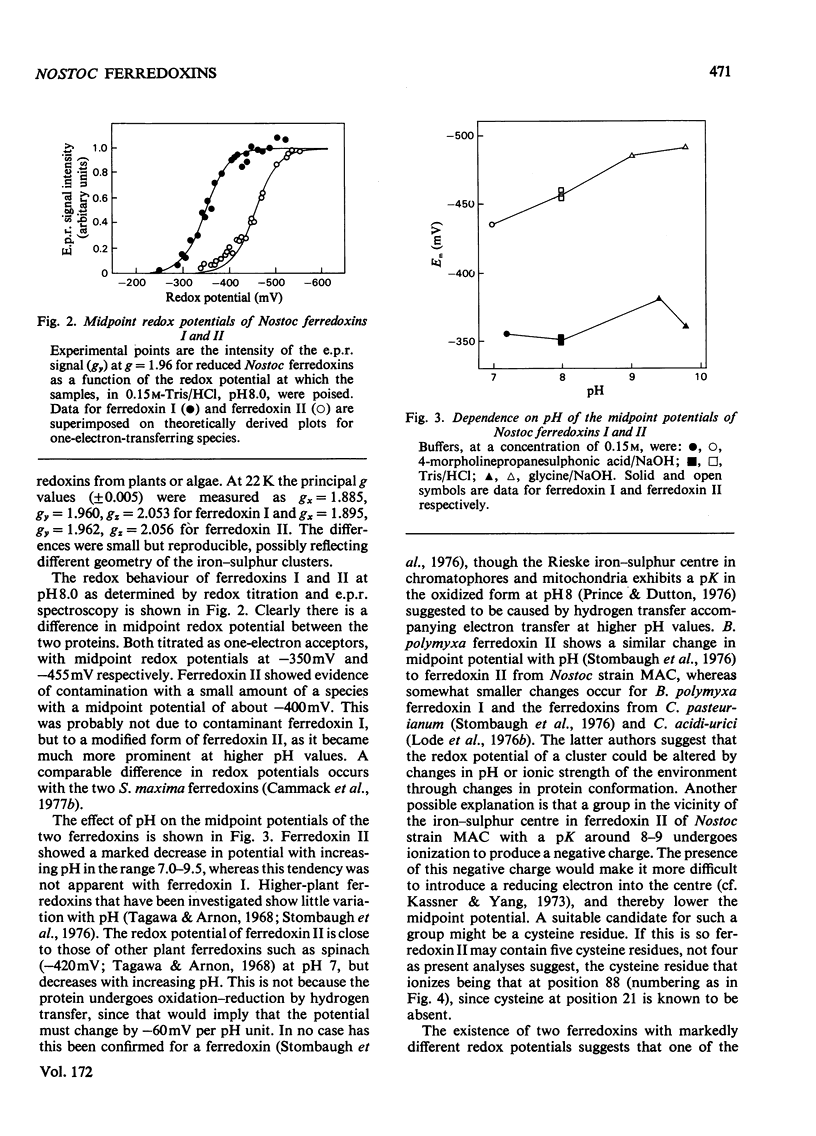
Two ferredoxins were isolated from the cyanobacterium Nostoc strain MAC grown autotrophically in the light or heterotrophically in the dark. In either case approximately three times as much ferredoxin I as ferredoxin II was obtained. Both ferredoxins had absorption maxima at 276, 282 (shoulder), 330, 423 and 465 nm in the oxidized state, and each possessed a single 2 Fe-2S active centre. Their isoelectric points were approx. 3.2. The midpoint redox potentials of the ferredoxins differed markedly; that of ferredoxin I was --350mV and that of ferredoxin II was --445mV, at pH 8.0. The midpoint potential of ferredoxin II was unusual in being pH dependent. Ferredoxin I was most active in supporting NADP+ photoreduction by chloroplasts, whereas ferredoxin II was somewhat more active in pyruvate decarboxylation by the phosphoroclastic system of Clostridum pasteurianum. Though the molecular weights of the ferredoxins determined by ultracentrifugation were the same within experimetnal error, the amino acid compositions showed marked differences. The N-terminal amino acid sequences of ferredoxins I and II were determined by means of an automatic sequencer. There are 11--12 differences between the sequences of the first 32 residues. It appears that the two ferredoxins have evolved separately to fulfil different roles in the organism.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal S. J., Rao K. K., Matsubara H. Horsetail ferredoxin: isolation and some chemical studies. J Biochem. 1971 Mar;69(3):601–603. [PubMed] [Google Scholar]
- Andrew P. W., Rogers L. J., Boulter D., Haslett B. G. Ferredoxin from a red alga, Porphyra umbilicalis. Eur J Biochem. 1976 Oct 1;69(1):243–248. doi: 10.1111/j.1432-1033.1976.tb10879.x. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. M., Yasunobu K. T. Non-heme iron proteins. X. The amino acid sequences of ferredoxins from Leucaena glauca. J Biol Chem. 1969 Feb 10;244(3):955–963. [PubMed] [Google Scholar]
- Bowyer J. W., Skerman V. B. Production of axemic cultures of soil-borne and endophytic blue-green algae. J Gen Microbiol. 1968 Dec;54(2):299–306. doi: 10.1099/00221287-54-2-299. [DOI] [PubMed] [Google Scholar]
- Bruschi M., Hatchikian C., Le Gall J., Moura J. J., Xavier A. V. Purification, characterization and biological activity of three forms of ferredoxin from the sulfate-reducing bacterium Desulfovibrio gigas. Biochim Biophys Acta. 1976 Nov 9;449(2):275–284. doi: 10.1016/0005-2728(76)90139-0. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Cammack R., Barber M. J., Bray R. C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem J. 1976 Aug 1;157(2):469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Bargeron C. P., Hutson K. G., Andrew P. W., Rogers L. J. Midpoint redox potentials of plant and algal ferredoxins. Biochem J. 1977 Nov 15;168(2):205–209. doi: 10.1042/bj1680205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Moura J. J., Xavier A. V., Bruschi M., Le Gall J., Deville A., Gayda J. P. Spectroscopic studies of the oxidation-reduction properties of three forms of ferredoxin from Desulphovibrio gigas. Biochim Biophys Acta. 1977 Feb 22;490(2):311–321. doi: 10.1016/0005-2795(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Franzen J. S., Braginski J. E. Oxidized triphosphopyridine nucleotide specific isocitrate dehydrogenase from Azotobacter vinelandii. Modification of a reactive sulfhydryl group with cyanide. Biochemistry. 1971 Jul 20;10(15):2872–2876. doi: 10.1021/bi00791a012. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Yasunobu K. T. Phylogenies from amino acid sequences aligned with gaps: the problem of gap weighting. J Mol Evol. 1975 Jun 9;5(1):1–24. doi: 10.1007/BF01732010. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K., Dovey T. A rapid and direct method for the quantitative determination of tryptophan in the intact protein. Biochem J. 1970 May;117(5):907–911. doi: 10.1042/bj1170907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickson J. D., Phillips W. D., McDonald C. C., Poe M. PMR characterization of alfalfa and soybean ferredoxins: the existence of two ferredoxins in soybean. Biochem Biophys Res Commun. 1971 Jan 22;42(2):271–279. doi: 10.1016/0006-291x(71)90098-2. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Cammack R., Rao K. K., Evans M. C., Mullinger R. Ferredoxins, blue-green bacteria and evolution. Biochem Soc Trans. 1975;3(3):361–368. doi: 10.1042/bst0030361. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. A stable and easily extractable plant-type ferredoxin from the blue-green alga Spirulina maxima. Biochem Biophys Res Commun. 1972 May 26;47(4):798–802. doi: 10.1016/0006-291x(72)90562-1. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. The iron-sulphur proteins: structure, function and evolution of a ubiquitous group of proteins. Sci Prog. 1975 Summer;62(246):285–317. [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. A minor component of ferredoxin from Aphanothece sacrum cells. J Biochem. 1975 Sep;78(3):605–610. doi: 10.1093/oxfordjournals.jbchem.a130946. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Amino acid sequence of the major component of Aphanothece sacrum ferredoxin. J Biochem. 1976 Feb;79(2):329–343. doi: 10.1093/oxfordjournals.jbchem.a131076. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Horsetail (Equisetum arvense) ferredoxins I and II Amino acid sequences and gene duplication. J Biochem. 1977 Jul;82(1):277–286. doi: 10.1093/oxfordjournals.jbchem.a131680. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Horsetail (Equisetum telmateia) ferredoxins I and II. Amino acid sequences. J Biochem. 1977 Jul;82(1):267–276. doi: 10.1093/oxfordjournals.jbchem.a131679. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Ohmiya M., Matsubara H. Amino acid sequence of the major component of Nostoc muscorum ferredoxin. J Biochem. 1976 Nov;80(5):993–999. doi: 10.1093/oxfordjournals.jbchem.a131387. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Matsubara H. Halobacterium halobium ferredoxin. A homologous protein to choroplast-type ferredoxins. FEBS Lett. 1977 May 15;77(2):308–310. doi: 10.1016/0014-5793(77)80257-3. [DOI] [PubMed] [Google Scholar]
- Haslett B. G., Boulter D. The N-terminal amino acid sequence of plastocyanin from Stellaria media L. An exercise to establish criteria for the identification of residues from a sequenator. Biochem J. 1976 Jan 1;153(1):33–38. doi: 10.1042/bj1530033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J. Two plant-type ferredoxins in light-grown and dark-grown cells of the blue-green bacterium Nostoc strain MAC. Biochem Soc Trans. 1975;3(3):377–379. doi: 10.1042/bst0030377. [DOI] [PubMed] [Google Scholar]
- Kagamiyama H., Rao K. K., Hall D. O., Cammack R., Matsubara H. Equisetum (horsetail) ferredoxin: characterization of the active centre and position of the four cysteine residues in this 2Fe-2S protein. Biochem J. 1975 Jan;145(1):121–123. doi: 10.1042/bj1450121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner R. J., Yang W. The redox potentials of the two-iron plant and algal ferredoxins. An electrostatic model. Biochem J. 1973 Jun;133(2):283–287. doi: 10.1042/bj1330283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Suzuki K. Components of the electron transport system in adrenal steroid hydroxylase. Isolation and properties of non-heme iron protein (adrenodoxin). J Biol Chem. 1967 Feb 10;242(3):485–491. [PubMed] [Google Scholar]
- Kwanyuen P., Wildman S. G. Nuclear DNA codes for Nicotiana ferredoxin. Biochim Biophys Acta. 1975 Sep 9;405(1):167–174. doi: 10.1016/0005-2795(75)90327-x. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Pyruvate: ferredoxin oxidoreductase and its activation by ATP in the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1971 Aug 6;245(1):165–174. doi: 10.1016/0005-2728(71)90019-3. [DOI] [PubMed] [Google Scholar]
- Lode E. T., Murray C. L., Rabinowitz J. C. Apparent oxidation-reduction potential of Clostridium acidi-urici ferredoxin. Effect of pH, ionic strength, and amino acid replacements. J Biol Chem. 1976 Mar 25;251(6):1683–1687. [PubMed] [Google Scholar]
- Lode E. T., Murray C. L., Rabinowitz J. C. Derivatives of Clostridium acidi-urici ferredoxin containing altered amino acid sequences. Semisynthetic synthesis, biological activity, and stability. J Biol Chem. 1976 Mar 25;251(6):1675–1682. [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Tsukihara T., Tahara H., Katsube Y., Matsu-Ura Y. Location of the iron-sulfur cluster in Spirulina platensis ferredoxin by x-ray analysis. J Biochem. 1977 Feb;81(2):529–531. doi: 10.1093/oxfordjournals.jbchem.a131486. [DOI] [PubMed] [Google Scholar]
- Prince R. G., Dutton P. L. Further studies on the Rieske iron-sulfur center in mitochondrial and photosynthetic systems: a pK on the oxidized form. FEBS Lett. 1976 May 15;65(1):117–119. doi: 10.1016/0014-5793(76)80634-5. [DOI] [PubMed] [Google Scholar]
- Ramírez J. M., Del Campo F. F., Paneque A., Losada M. Ferredoxin-nitrite reductase from spinach. Biochim Biophys Acta. 1966 Apr 12;118(1):58–71. doi: 10.1016/s0926-6593(66)80144-3. [DOI] [PubMed] [Google Scholar]
- Shanmugam K. T., Buchanan B. B., Arnon D. I. Ferredoxins in light- and dark-grown photosynthetic cells with special reference to Rhodospirillum rubrum. Biochim Biophys Acta. 1972 Feb 28;256(2):477–486. doi: 10.1016/0005-2728(72)90076-x. [DOI] [PubMed] [Google Scholar]
- Shin M., Sukenobu M., Oshino R., Kitazume Y. Two plant-type ferredoxins from a blue-green alga, Nostoc verrucosum. Biochim Biophys Acta. 1977 Apr 11;460(1):85–93. doi: 10.1016/0005-2728(77)90154-2. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Stombaugh N. A., Sundquist J. E., Burris R. H., Orme-Johnson W. H. Oxidation-reduction properties of several low potential iron-sulfur proteins and of methylviologen. Biochemistry. 1976 Jun 15;15(12):2633–2641. doi: 10.1021/bi00657a024. [DOI] [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- Tagawa K., Arnon D. I. Oxidation-reduction potentials and stoichiometry of electron transfer in ferredoxins. Biochim Biophys Acta. 1968 Apr 2;153(3):602–613. doi: 10.1016/0005-2728(68)90188-6. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Zeitlin S., Yasunobu K. T., Evans M. C., Rao K. K., Hall D. O. Amino acid sequence of the Spirulina maxima ferredoxin, a ferredoxin from a procaryote. Biochem Biophys Res Commun. 1975 May 5;64(1):399–407. doi: 10.1016/0006-291x(75)90267-3. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Cammack R., Rao K. K., Rogers L. J., Stewart W. D., Hall D. O. Comparative immunochemistry of bacterial, algal and plant ferredoxins. Biochim Biophys Acta. 1977 Jan 25;490(1):120–131. doi: 10.1016/0005-2795(77)90112-x. [DOI] [PubMed] [Google Scholar]
- Van Baalen C. The effects of ultraviolet irradiation on a coccoid blue-green alga: survival, photosynthesis, and photoreactivation. Plant Physiol. 1968 Oct;43(10):1689–1695. doi: 10.1104/pp.43.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Hase T., Matsubara H. Evolutionary information involved in primary structures of chloroplast-type ferredoxins. J Biochem. 1975 Sep;78(3):637–639. doi: 10.1093/oxfordjournals.jbchem.a130950. [DOI] [PubMed] [Google Scholar]
- Wada K., Hase T., Tokunaga H., Matsubara H. Amino acid sequence of Spirulina platensis ferredoxin: a far divergency of blue-green algal ferredoxins. FEBS Lett. 1975 Jul 15;55(1):102–104. doi: 10.1016/0014-5793(75)80969-0. [DOI] [PubMed] [Google Scholar]
- Wada K., Kagamiyama H., Shin M., Matsubara H. Ferredoxin from a blue-green alga, Aphanothece sacrum (Suringar) Okada. J Biochem. 1974 Dec;76(6):1217–1225. doi: 10.1093/oxfordjournals.jbchem.a130674. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Takenami S., Wada K., Okunuki K. Purification and some properties of ferredoxin derived from the blue-green alga, Anacystis nidulans. Biochim Biophys Acta. 1969 May;180(1):196–198. doi: 10.1016/0005-2728(69)90208-4. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Comparison of two ferredoxins from Rhodospirillum rubrum as electron carriers for the native nitrogenase. J Bacteriol. 1975 Feb;121(2):743–745. doi: 10.1128/jb.121.2.743-745.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I., Sweeney W. V. Characterization of two soluble ferredoxins as distinct from bound iron-sulfur proteins in the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1975 Nov 10;250(21):8330–8336. [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]
- Yoch D. C. Purification and properties of two ferredoxins from the nitrogen-fixing bacterium Bacillus polymyxa. Arch Biochem Biophys. 1973 Oct;158(2):633–640. doi: 10.1016/0003-9861(73)90555-9. [DOI] [PubMed] [Google Scholar]


