Abstract
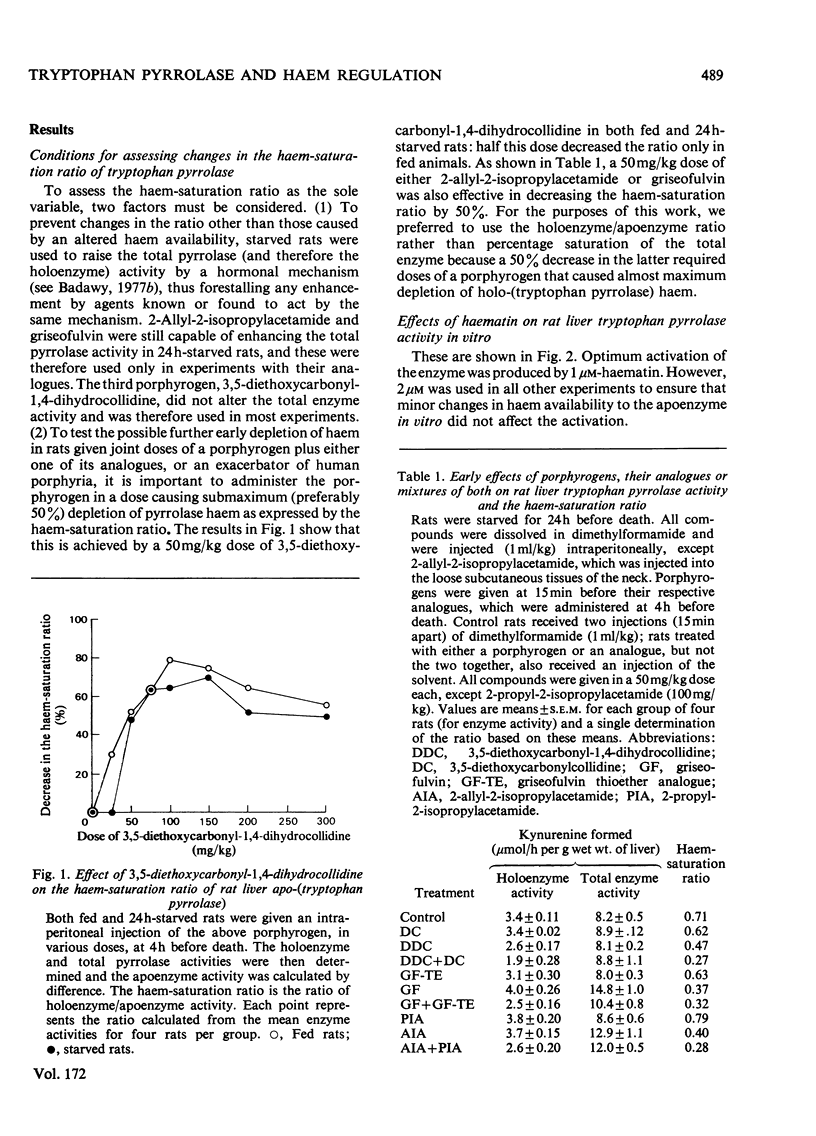
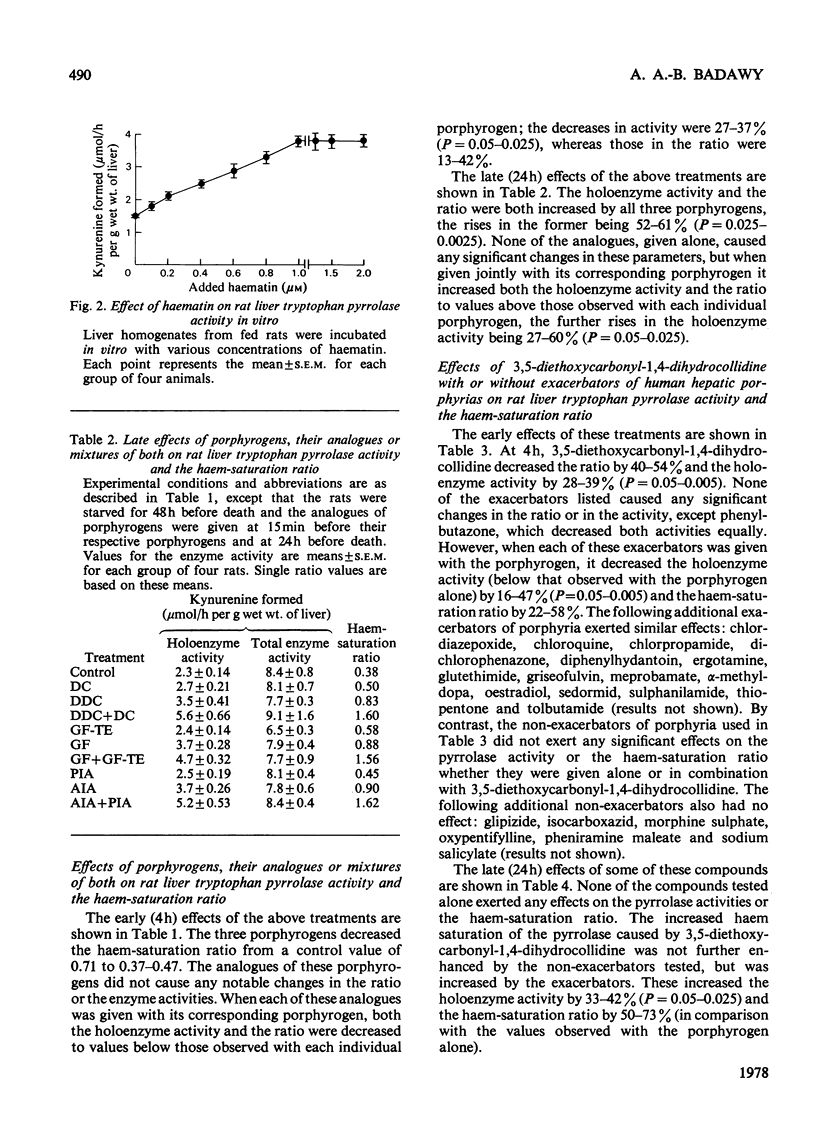
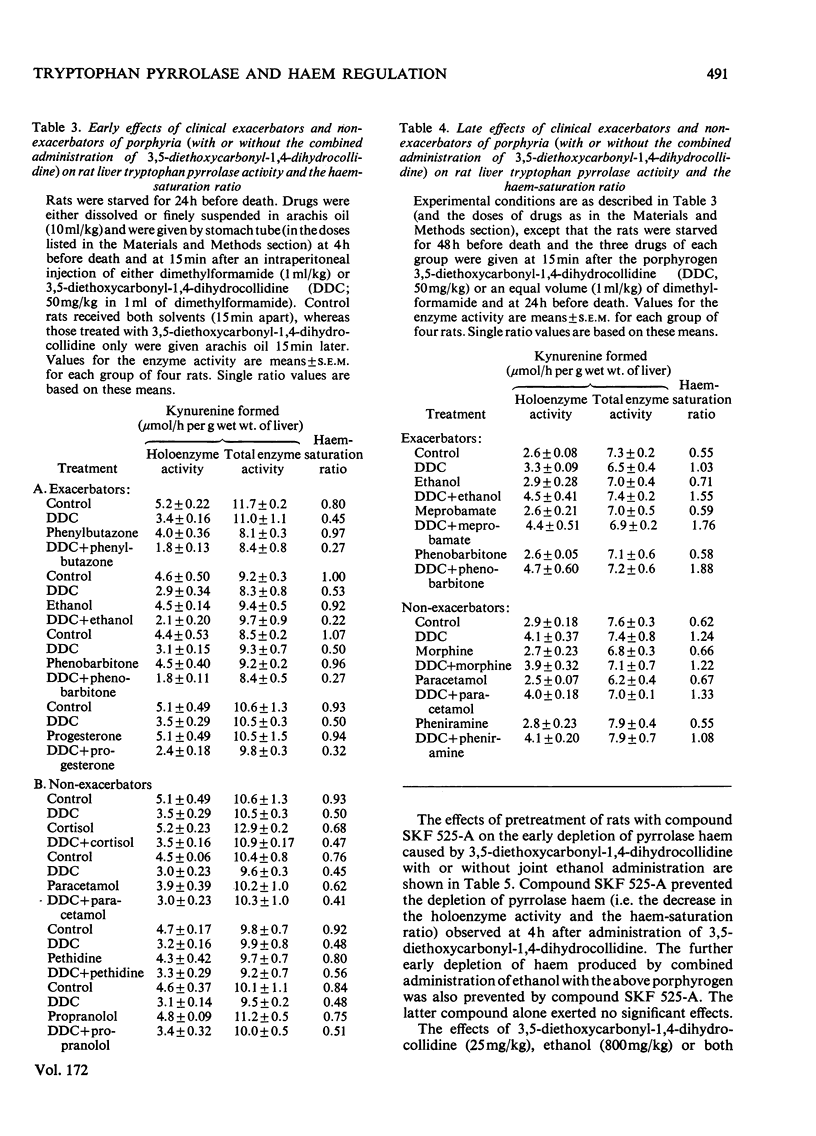
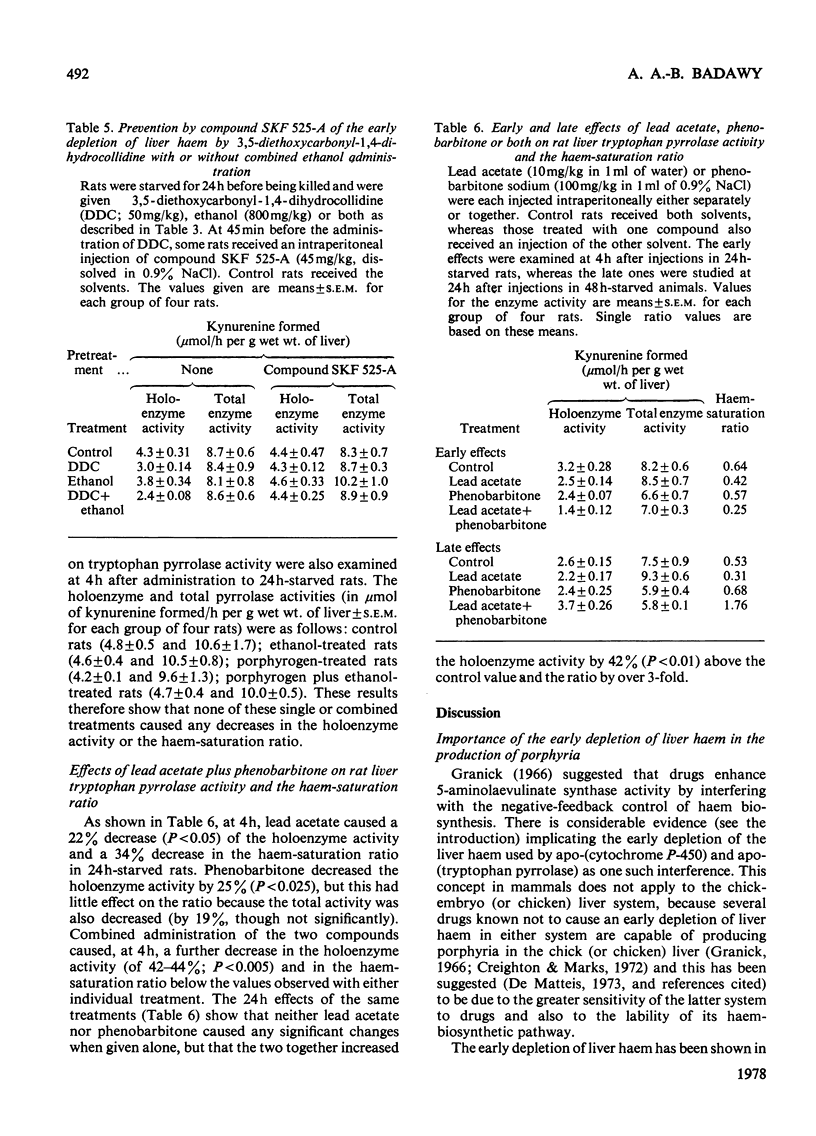
1. The importance of the early depletion of liver haem in the production of porphyria is discussed and further supporting evidence is presented from experiments with tryptophan pyrrolase, under conditions of exacerbation of experimental porphyria by therapeutic and other agents. 2. In addition to the early depletion of pyrrolase haem by porphyrogens, a further depletion is produced when rats are given a porphyrogen plus an analogue or one of 19 drugs known to exacerbate the human disease. 3. Non-exacerbators of human porphyrias do not cause a further early depletion of pyrrolase haem and it is suggested that this system may be used as a screening test for possible exacerbation of the disease by new and existing drugs. 4. A similar further early depletion of haem is produced by combined administration of lead acetate plus phenobarbitone, thus suggesting that the depletion is a more general phenomenon in experimental porphyria. 5. The relationship between tryptophan pyrrolase and the regulatory free haem is discussed. It is suggested that pyrrolase may play an important role in the regulation of haem biosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawy A. A., Evans M. Animal liver tryptophan pyrrolases: Absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976 Jul 15;158(1):79–88. doi: 10.1042/bj1580079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: Experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975 Sep;150(3):511–520. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of chemical porphyrogens and drugs on the activity of rat liver tryptophan pyrrolase. Biochem J. 1973 Dec;136(4):885–892. doi: 10.1042/bj1360885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. The effects of acetate, metal cations, phenobarbitone, porphyrogens and substrates of glycine acyltransferase on the utilization of haem by rat liver apo-(tryptophan pyrrolase). Biochem J. 1977 May 15;164(2):431–438. doi: 10.1042/bj1640431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. The functions and regulation of tryptophan pyrrolase. Life Sci. 1977 Sep 15;21(6):755–768. doi: 10.1016/0024-3205(77)90402-7. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Weiner R., Fröhling W. Regulation of delta-aminolevulinic acid synthetase by drugs and steroids in isolated perfused rat liver. Enzyme. 1973;16(1):295–301. doi: 10.1159/000459393. [DOI] [PubMed] [Google Scholar]
- Creighton J. M., Marks G. S. Drug-induced porphyrin biosynthesis. VII. Species, sex, and developmental differences in the generation of experimental porphyria. Can J Physiol Pharmacol. 1972 Jun;50(6):485–489. doi: 10.1139/y72-074. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Drug interactions in experimental hepatic porphyria. A model for the exacerbation by drugs of human variegate porphyria. Enzyme. 1973;16(1):266–275. doi: 10.1159/000459390. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Stimulation of the pathway of porphyrin synthesis in the liver of rats and mice by griseofulvin, 3,5-Diethoxycarbonyl-1,4-dihydrocollidine and related drugs: evidence for two basically different mechanisms. Biochem J. 1975 Jan;146(1):285–287. doi: 10.1042/bj1460285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. Stimulation of liver 5-aminolaevulinate synthetase by drugs and its relevance to drug-induced accumulation of cytochrome P-450. Studies with phenylbutazone and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Biochem J. 1972 Mar;126(5):1149–1160. doi: 10.1042/bj1261149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. The effect of drugs on 5-aminolaevulinate synthetase and other enzymes in the pathway of liver haem biosynthesis. Basic Life Sci. 1975;6:185–205. doi: 10.1007/978-1-4615-8954-9_7. [DOI] [PubMed] [Google Scholar]
- Dhar G. J., Bossenmaier I., Petryka Z. J., Cardinal R., Watson C. J. Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975 Jul;83(1):20–30. doi: 10.7326/0003-4819-83-1-20. [DOI] [PubMed] [Google Scholar]
- FEIGELSON P., GREENGARD O. A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase. J Biol Chem. 1961 Jan;236:153–157. [PubMed] [Google Scholar]
- FOUTS J. R., BRODIE B. B. Inhibition of drug metabolic pathways by the potentiating agent, 2, 4-dichloro-6-phenyl-phenoxyethyl diethylamine. J Pharmacol Exp Ther. 1955 Sep;115(1):68–73. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Israels L. G., Yoda B., Schacter B. A. Heme binding and its possible significance in heme movement and availability in the cell. Ann N Y Acad Sci. 1975 Apr 15;244:651–661. doi: 10.1111/j.1749-6632.1975.tb41559.x. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Srai K. S., Christodoulides L. Haem-binding proteins of the rat liver cytosol. Biochim Biophys Acta. 1976 May 28;428(3):683–689. doi: 10.1016/0304-4165(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Rao M. R., Malathi K., Padmanaban G. The relationship between delta-aminolaevulinate synthetase induction and the concentration of cytochrome P-450 and catalase in rat liver. Biochem J. 1972 Apr;127(3):553–559. doi: 10.1042/bj1270553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda B., Israels L. G. Transfer of heme from mitochondria in rat liver cells. Can J Biochem. 1972 Jun;50(6):633–637. doi: 10.1139/o72-087. [DOI] [PubMed] [Google Scholar]
- de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967 Dec;19(4):523–557. [PubMed] [Google Scholar]


