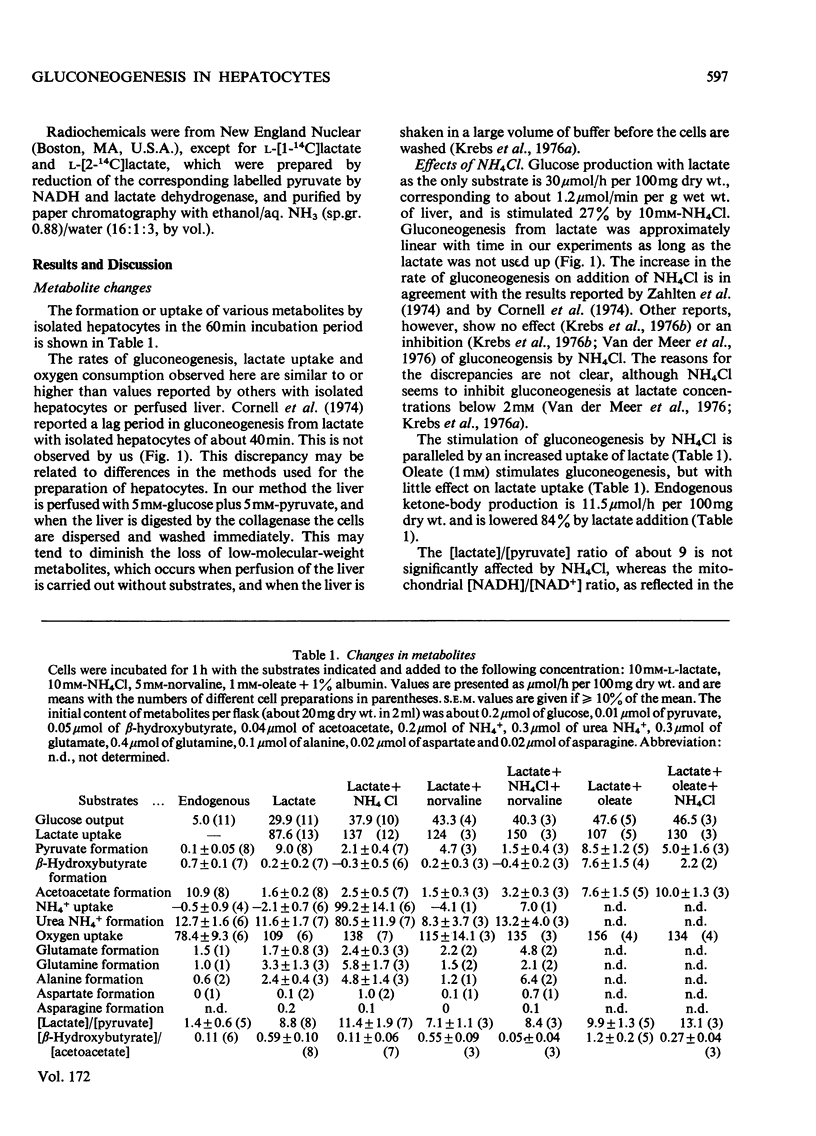
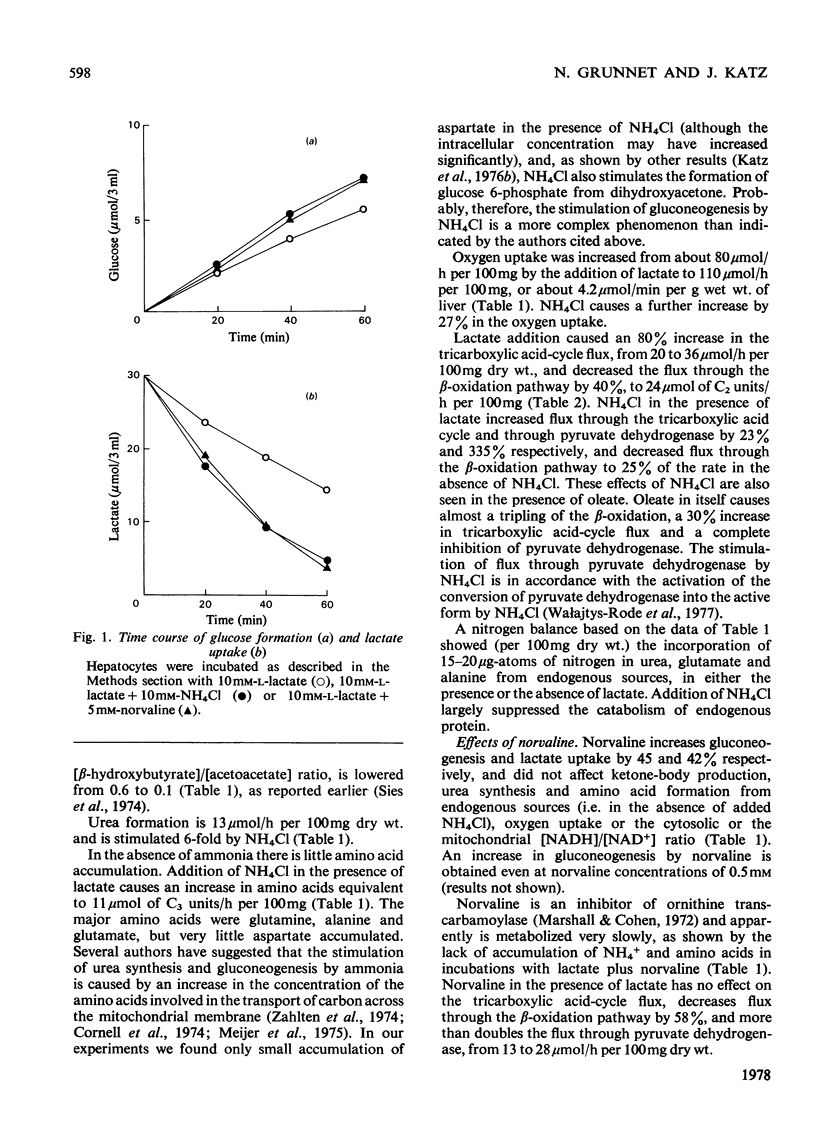
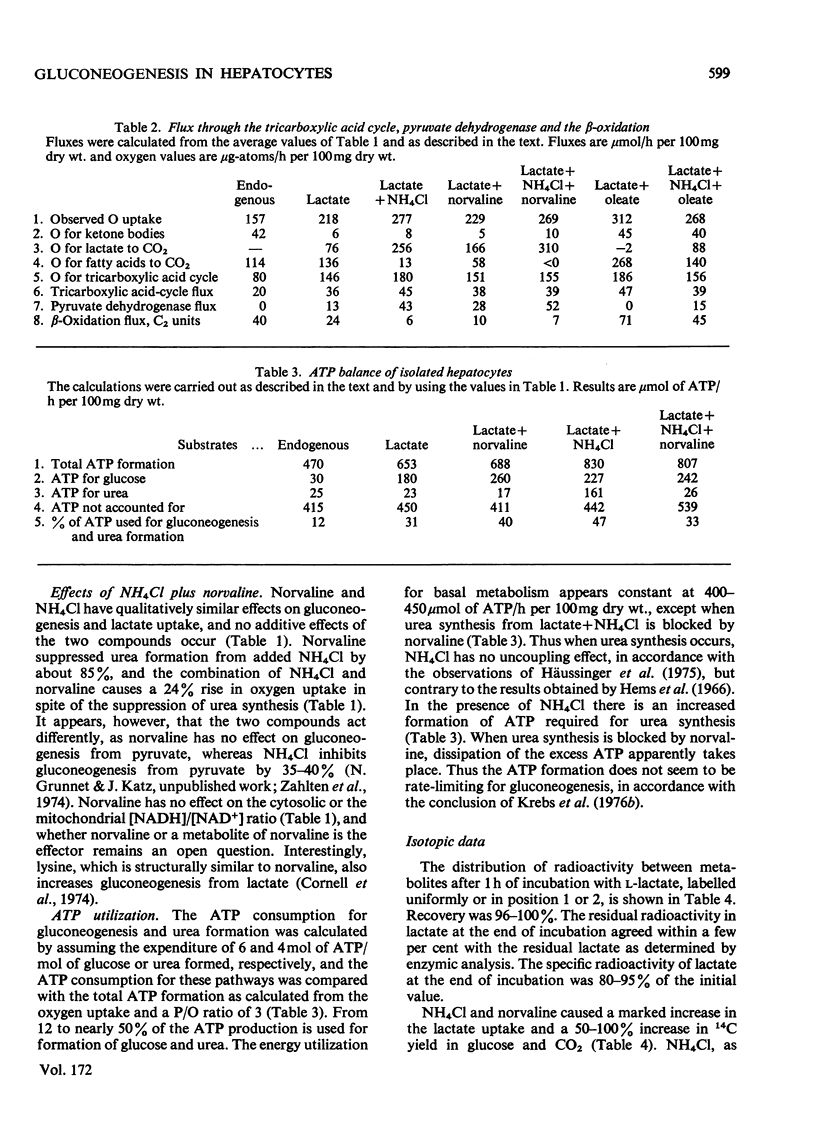
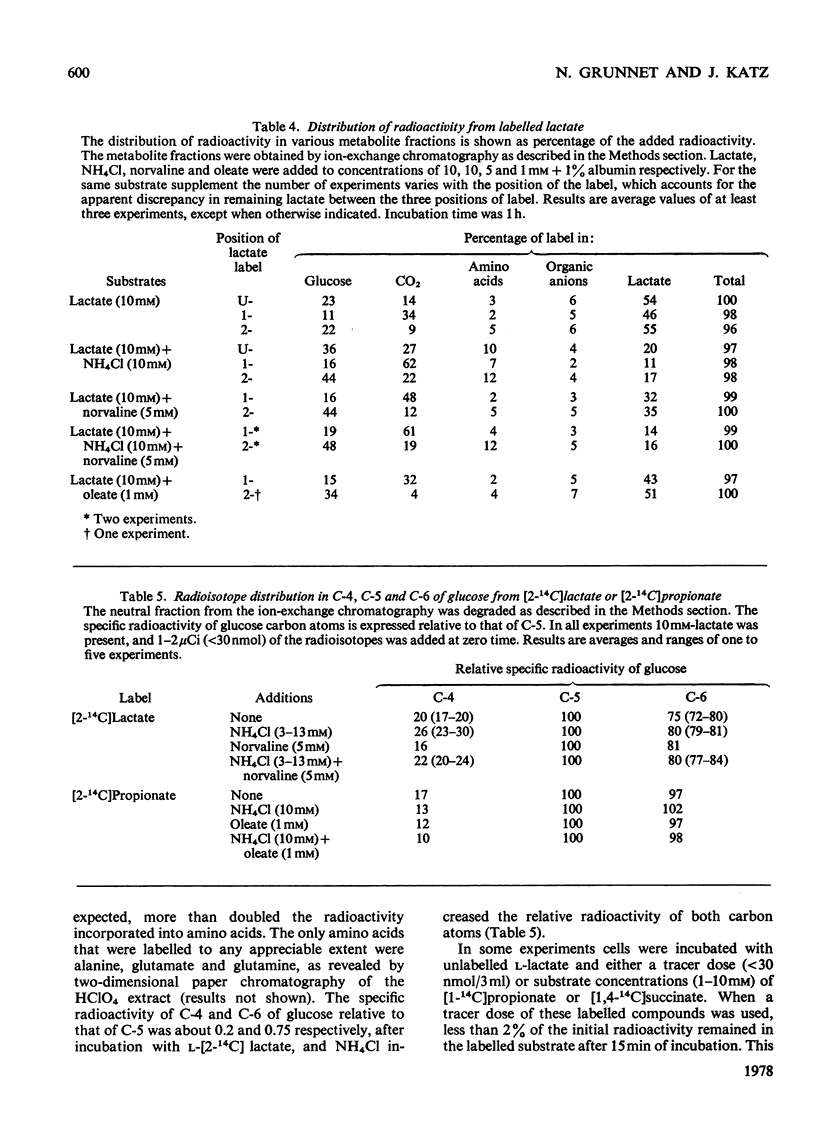
Abstract
1. Hepatocytes from starved rats were incubated with l-lactate and NH4Cl or norvaline, and the rates of the tricarboxylic acid cycle and of gluconeogenesis were calculated from changes in metabolite concentrations or from radioisotopic data from incubations with labelled lactate or propionate. 2. Gluconeogenesis was stimulated by the addition of 10mm-NH4Cl, 5mm-norvaline or 1mm-oleate by 27, 45 and 59% respectively. NH4Cl or norvaline also increased lactate uptake. Norvaline inhibited urea synthesis from NH4Cl by 85%. 3. The effects of NH4Cl and norvaline were not additive. However, NH4Cl inhibited and norvaline was without effect on gluconeogenesis from pyruvate, indicating that the two compounds act by different mechanisms. 4. The tricarboxylic acid-cycle flux was increased 80% by lactate, and NH4Cl caused a further 25% stimulation. Norvaline had no effect on the tricarboxylic acid-cycle flux. NH4Cl and norvaline tripled and doubled, respectively, flux through pyruvate dehydrogenase. 5. Total ATP formation was calculated to range from 470 to 830μmol/h per 100mg of protein, of which the basic metabolic activity accounted for 400–450μmol/h per 100mg of protein. ATP formation does not seem to be rate-limiting for gluconeogenesis. 6. Pyruvate recycling was estimated from the 14C yield from [1-14C]propionate in lactate and glucose to be 10–30% of the flux of phosphoenolpyruvate to glucose. The further addition of NH4Cl more than doubled the recycling of pyruvate. 7. [1,4-14C]Succinate was rapidly metabolized by hepatocytes. About 20% of the radioactivity was recovered in glucose, indicating that succinate is also metabolized by intact (non-damaged) hepatocytes. 8. It is concluded that the metabolism of lactate by the liver is too complex to allow simple rate measurements with labelled compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Harper S. C. Control of gluconeogenesis in liver. V. Effects of fasting, diabetes, and glucagon on lactate and endogenous metabolism in the perfused rat liver. J Biol Chem. 1972 Aug 25;247(16):4996–5003. [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr, Saunders H. L., Kostos V., Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys. 1971 Apr;143(2):566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Weiss L., Sies H. Activation of pyruvate dehydrogenase during metabolism of ammonium ions in hemoglobin-free perfused rat liver. Eur J Biochem. 1975 Apr 1;52(3):421–431. doi: 10.1111/j.1432-1033.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- KATZ J., CHAIKOFF I. L. A chromatographic-radioautographic method for study of acetate utilization in animal tissues. J Biol Chem. 1954 Feb;206(2):887–900. [PubMed] [Google Scholar]
- Katz J., Golden S., Dunn A., Chenoweth M. Estimation of glucose turnover in rats in vivo with tritium labeled glucoses. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1387–1394. doi: 10.1515/bchm2.1976.357.2.1387. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Tyler B. The regulation of folate and methionine metabolism. Biochem J. 1976 Aug 15;158(2):341–353. doi: 10.1042/bj1580341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes J. P., Harris R. A. On the oxidation of succinate by parenchymal cells isolated from rat liver. FEBS Lett. 1975 Mar 1;51(1):80–83. doi: 10.1016/0014-5793(75)80858-1. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G. A., Tager J. M., Williamson J. R. Role of anion translocation across the mitochondrial membrane in the regulation of urea synthesis from ammonia by isolated rat hepatocytes. J Biol Chem. 1975 Oct 10;250(19):7728–7738. [PubMed] [Google Scholar]
- Müllhofer G., Müller C., Von Stetten C., Gruber E. Carbon-14 tracer studies in the metabolism of isolated rat-liver parenchymal cells under conditions of gluconeogenesis from lactate and pyruvate. Eur J Biochem. 1977 May 16;75(2):331–341. doi: 10.1111/j.1432-1033.1977.tb11533.x. [DOI] [PubMed] [Google Scholar]
- Müllhofer G., Schwab A., Müller C., Von Stetten C., Gruber E. Carbon-14 tracer studies in rat-liver perfusion experiments under conditions of gluconeogenesis from lactate and pyruvate. Eur J Biochem. 1977 May 16;75(2):319–330. doi: 10.1111/j.1432-1033.1977.tb11532.x. [DOI] [PubMed] [Google Scholar]
- Nakai T., Otto P. S., Kennedy D. L., Whayne T. F., Jr Rat high density lipoprotein subfraction (HDL3) uptake and catabolism by isolated rat liver parenchymal cells. J Biol Chem. 1976 Aug 25;251(16):4914–4921. [PubMed] [Google Scholar]
- Rognstad R. Cyclic AMP induced inhibition of pyruvate kinase flux in the intact liver cell. Biochem Biophys Res Commun. 1975 Apr 21;63(4):900–905. doi: 10.1016/0006-291x(75)90653-1. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J Biol Chem. 1977 Mar 25;252(6):1831–1833. [PubMed] [Google Scholar]
- Rognstad R. Sources of ammonia for urea synthesis in isolated rat liver cells. Biochim Biophys Acta. 1977 Feb 28;496(2):249–254. doi: 10.1016/0304-4165(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Genovese J., Katz J. Enzymic degradation of isotopically labeded compounds. II. Glucose labeled with 14C and tritium. Anal Biochem. 1970 Mar;34:170–179. doi: 10.1016/0003-2697(70)90098-9. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Genovese J., Katz J. Enzymic degradation of isotopically labeded compounds. II. Glucose labeled with 14C and tritium. Anal Biochem. 1970 Mar;34:170–179. doi: 10.1016/0003-2697(70)90098-9. [DOI] [PubMed] [Google Scholar]
- Sies H., Häussinger D., Grosskopf M. Mitochondrial nicotinamide nucleotide systems: ammonium chloride responses and associated metabolic transitions in hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):305–320. doi: 10.1515/bchm2.1974.355.1.305. [DOI] [PubMed] [Google Scholar]
- Spencer T. L. The transport and oxidation of succinate by Ehrlich ascites-tumour cells. Biochem J. 1976 Oct 15;160(1):121–123. doi: 10.1042/bj1600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T. Control mechanisms of gluconeogenesis and ketogenesis. II. Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4617–4627. [PubMed] [Google Scholar]
- Zahlten R. N., Kneer N. M., Stratman F. W., Lardy H. A. The influence of ammonium and calcium lons on gluconeogenesis in isolated rat hepatocytes and their response to glucagon and epinephrine. Arch Biochem Biophys. 1974 Apr 2;161(2):528–535. doi: 10.1016/0003-9861(74)90335-x. [DOI] [PubMed] [Google Scholar]


