Abstract
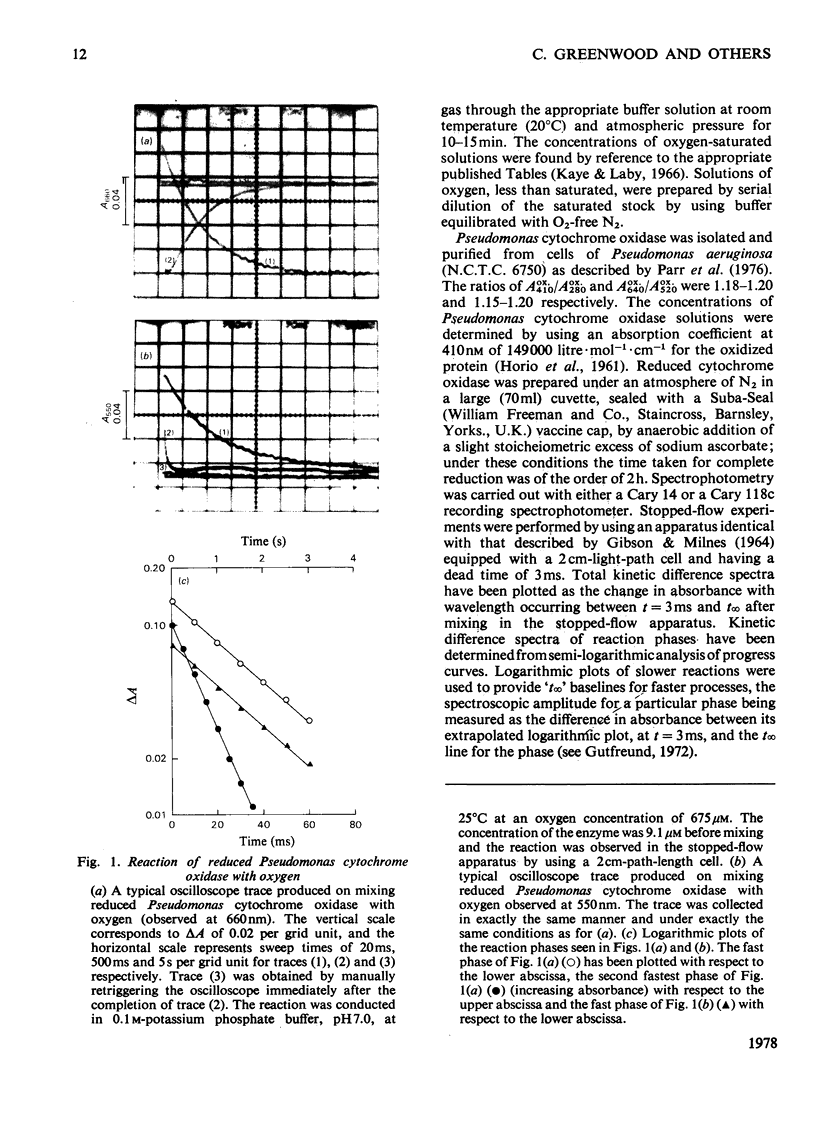
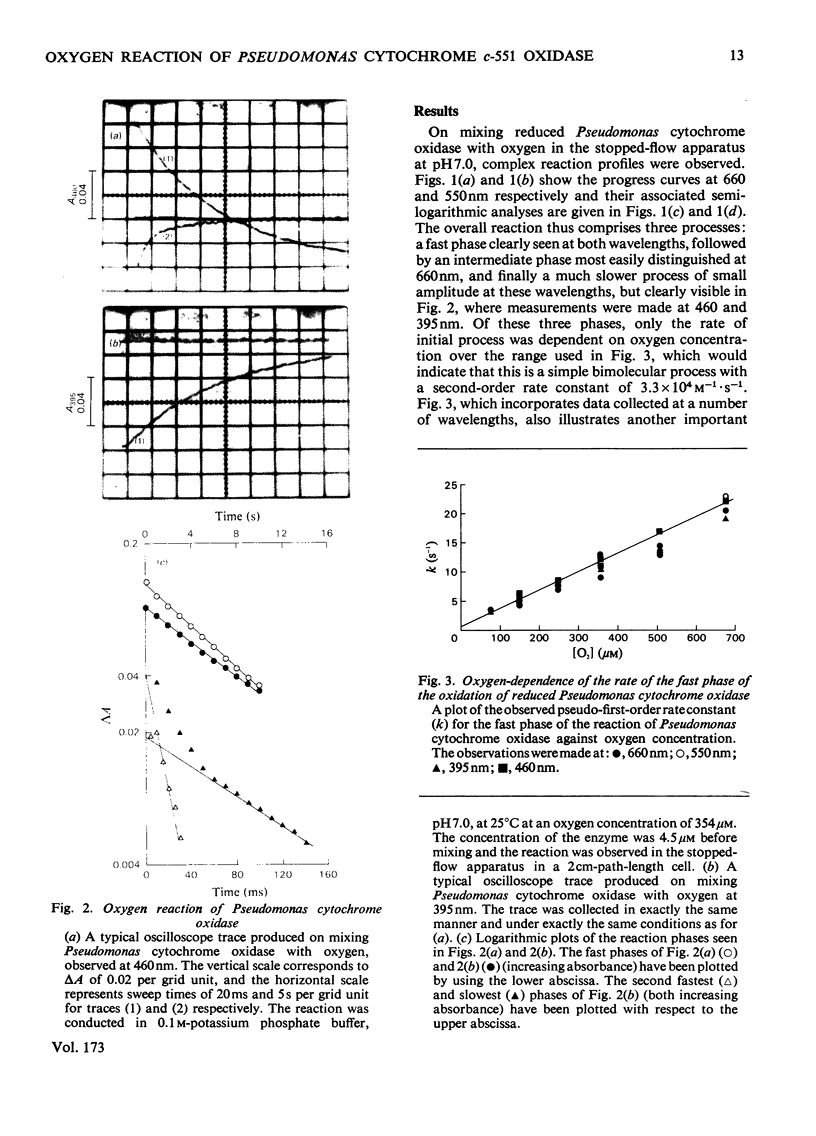
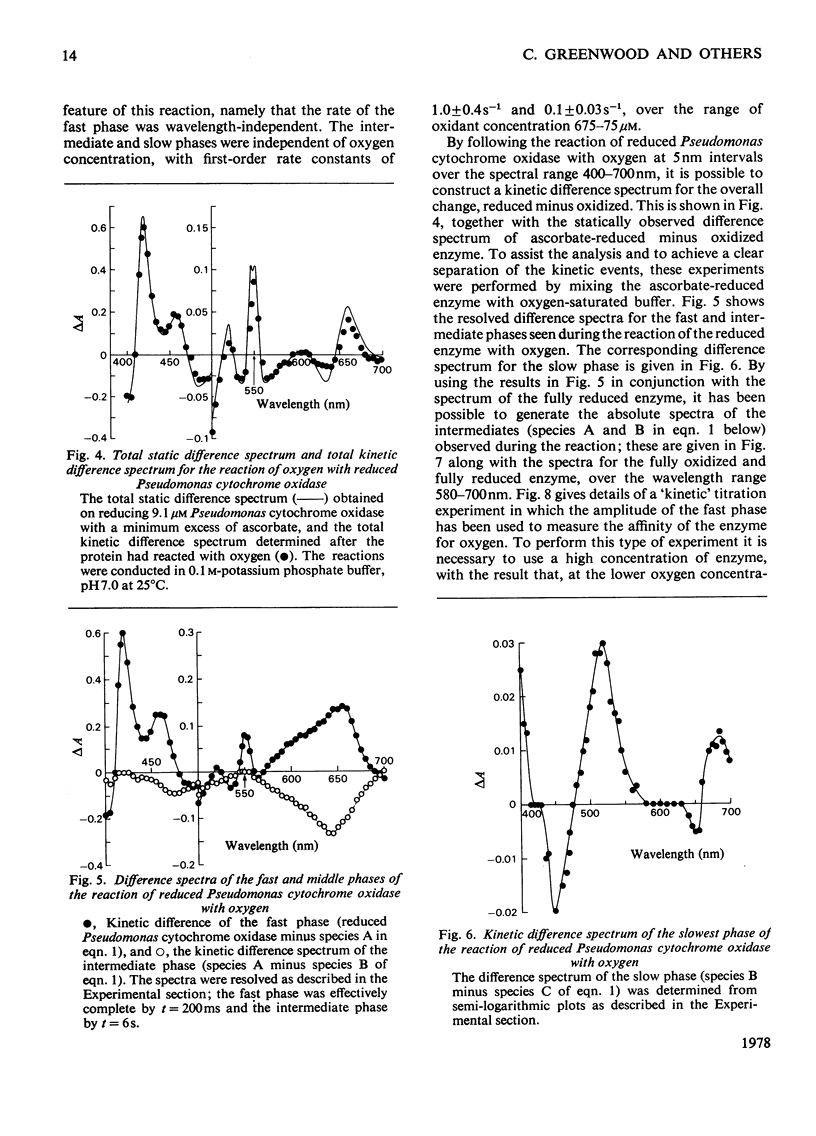
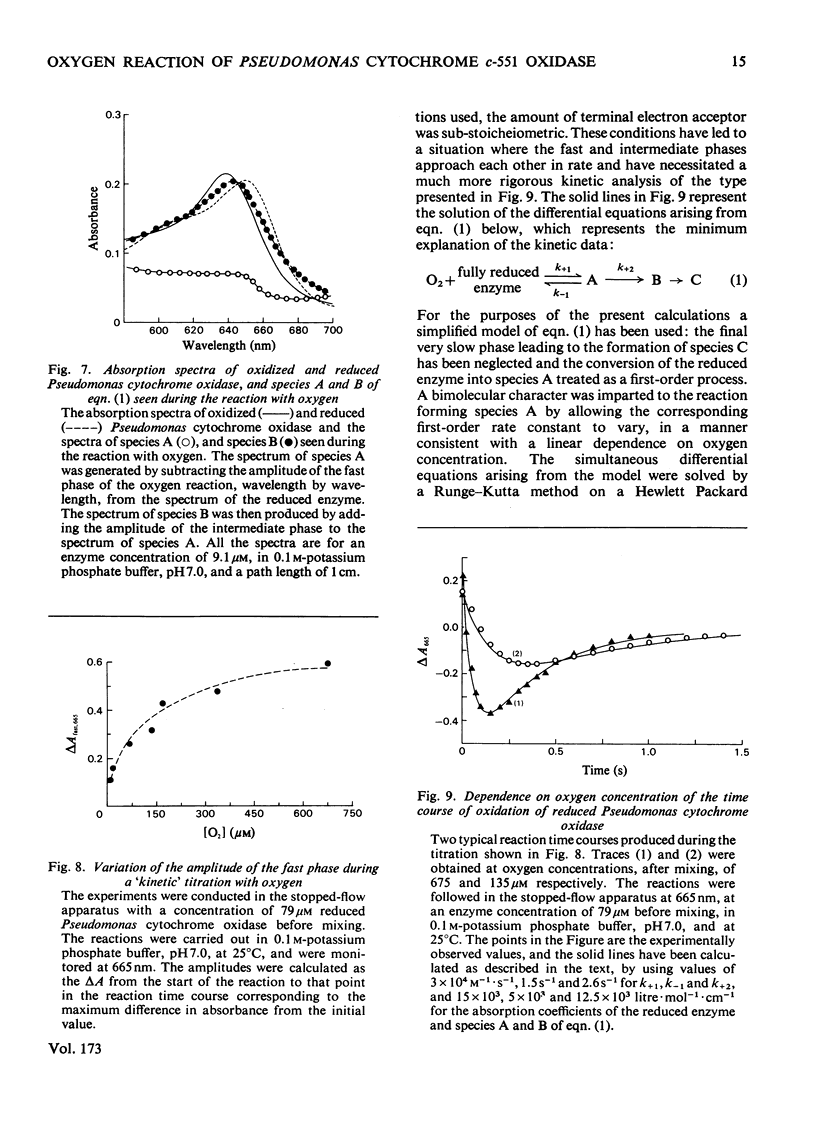
The reaction of ascorbate-reduced Pseudomonas cytochrome oxidase with oxygen was studied by using stopped-flow techniques at pH 7.0 and 25 degrees C. The observed time courses were complex, the reaction consisting of three phases. Of these, only the fastest process, with a second-order rate constant of 3.3 X 10(4) M-1.S-1, was dependent on oxygen concentration. The two slower processes were first-order reactions with rates of 1.0 +/- 0.4s-1 and 0.1 +/- 0.03s-1. A kinetic titration experiment revealed that the enzyme had a relatively low affinity constant for oxygen, approx. 10(4)M-1. Kinetic difference spectra were determined for all three reaction phases, showing each to have different characteristics. The fast-phase difference spectrum showed that changes occurred at both the haem c and haem d1 components of the enzyme during this process. These changes were consistent with the haem c becoming oxidized, but with the haem d1 assuming a form that did not correspond to the normal oxidized state, a situation that was not restored even after the second kinetic phase, which reflected further changes in the haem d1 component. The results are discussed in terms of a kinetic scheme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D., Parr S. R., Greenwood C. The reduction of Pseudomonas cytochrome c551 oxidase by chromous ions. Biochem J. 1977 Jun 1;163(3):629–632. doi: 10.1042/bj1630629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Gibson Q. H. The reaction of reduced cytochrome C oxidase with oxygen. J Biol Chem. 1967 Apr 25;242(8):1782–1787. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., MATSUBARA H., KUSAI K., NAKAI M., OKUNUKI K. High purification and properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1958 Aug;29(2):297–302. doi: 10.1016/0006-3002(58)90188-4. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Kuronen T., Ellfolk N. A new purification procedure and molecular properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1972 Sep 20;275(3):308–318. doi: 10.1016/0005-2728(72)90212-5. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C., Brunori M. The electron-transfer reaction between azurin and the cytochrome c oxidase from Pseudomonas aeruginosa. Biochem J. 1977 Nov 1;167(2):447–455. doi: 10.1042/bj1670447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeruginosa cytochrome c oxidase with carbon monoxide. Biochem J. 1975 Oct;151(1):51–59. doi: 10.1042/bj1510051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Orii Y. Oxidation-reduction behavior of the heme c and heme d moieties of Pseudomonas aeruginosa nitrite reductase and the formation of an oxygenated intermediate at heme d1. J Biochem. 1976 Jul;80(1):135–140. doi: 10.1093/oxfordjournals.jbchem.a131245. [DOI] [PubMed] [Google Scholar]
- Wharton D. C., Gibson Q. H. Cytochrome oxidase from Pseudomonas aeruginosa. IV. Reaction with oxygen and carbon monoxide. Biochim Biophys Acta. 1976 Jun 8;430(3):445–453. doi: 10.1016/0005-2728(76)90020-7. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., KIJIMOTO S., OKUNUKI K. Biological significance of Pseudomonas cytochrome oxidase in Pseudomonas aeruginosa. J Biochem. 1963 May;53:416–421. doi: 10.1093/oxfordjournals.jbchem.a127716. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. Crystalline Pseudomonas cytochrome oxidase. II. Spectral properties of the enzyme. Biochim Biophys Acta. 1963 Mar 12;67:394–406. doi: 10.1016/0006-3002(63)91845-6. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. Activation of Pseudomonas cytochrome oxidase by catalase. J Biochem. 1961 May;49:414–420. doi: 10.1093/oxfordjournals.jbchem.a127319. [DOI] [PubMed] [Google Scholar]