Abstract
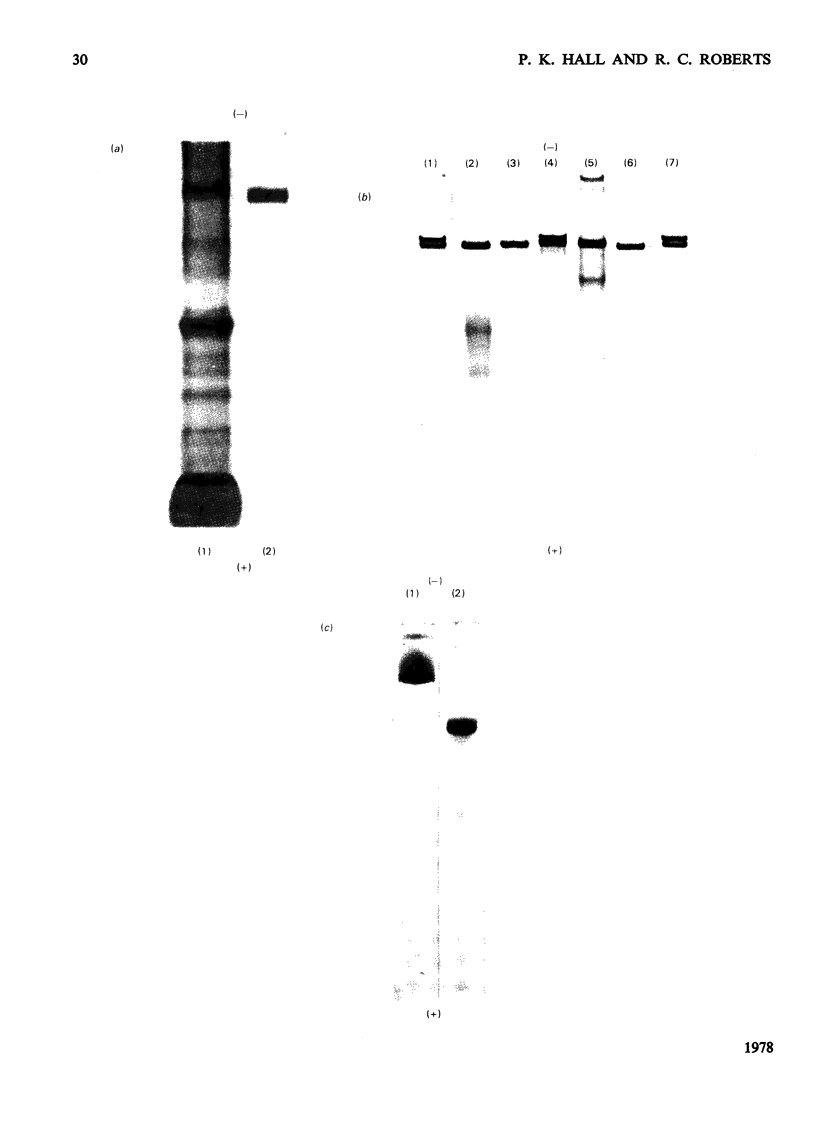
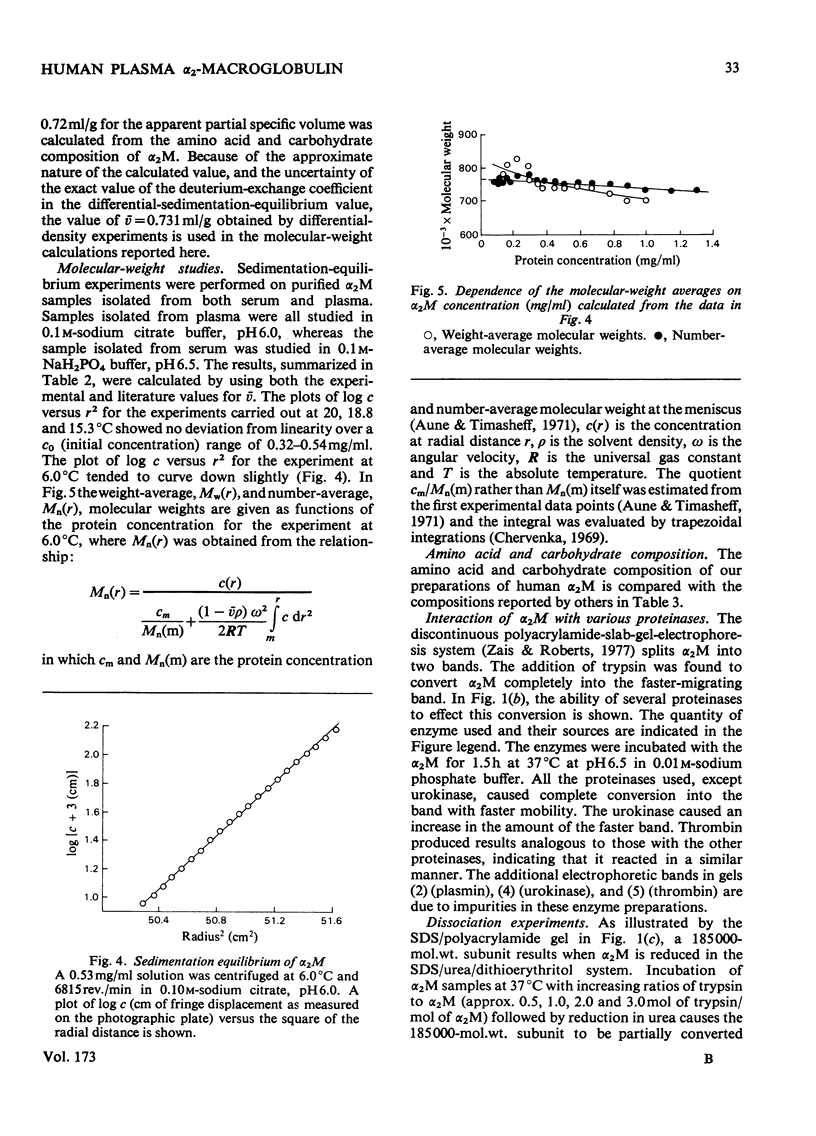
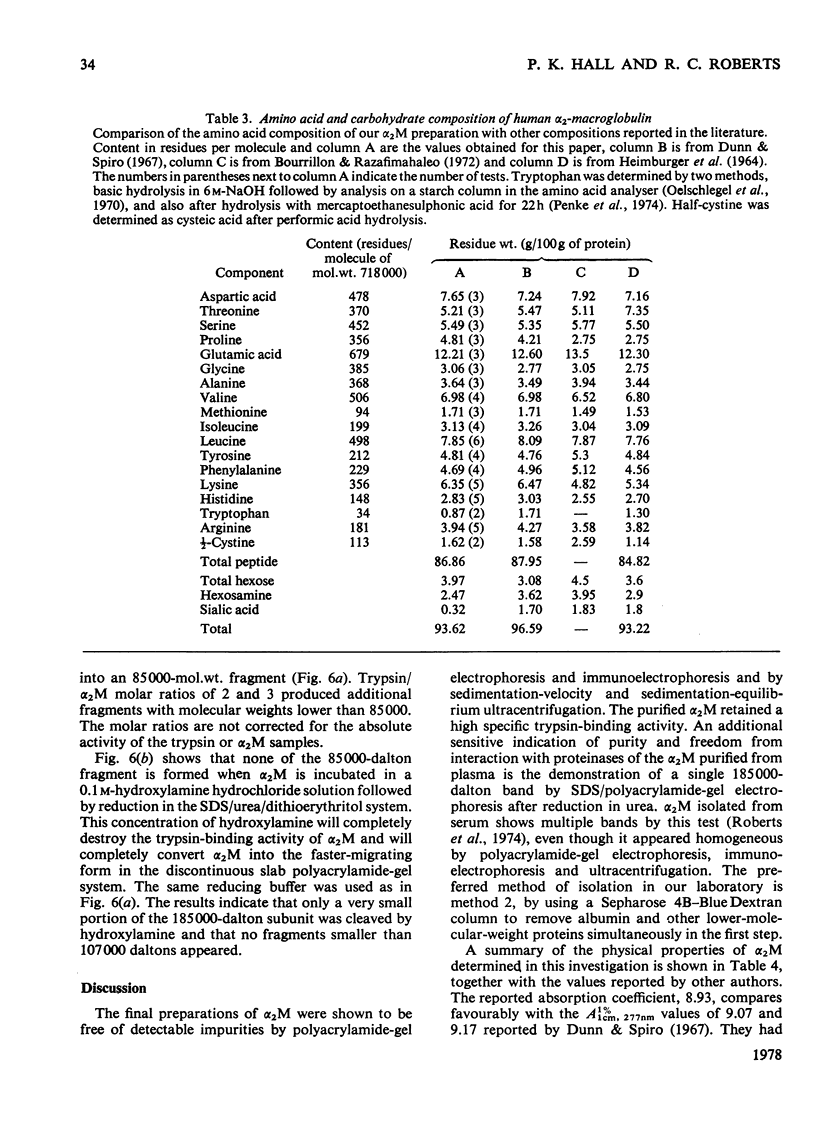
Alpha2-M (alpha2-macroglobulin) was purified from human plasma by two different procedures. As well as having no detectable impurities by the usual criteria for testing the homogeneity of protein preparations, these alpha2M preparations showed a single component, after reduction in urea, of 185000 daltons by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The molecular weight of the alpha2M was found to be 718000 by sedimentation equilibrium experiments using the gravimetrically determined -v of 0.731 ml/g. The interaction of several proteinases with alpha2M was studied by using a novel discontinuous polyacrylamide-gel system, which showed clear separation of the enzyme-complexed alpha2M from the free alpha2M. These studies indicated that urokinase, as well as trypsin, chymotrypsin, plasmin and thrombin forms complexes with alphaM. The cleavage of the 185000-dalton subunit to a 85000-dalton species on interaction of trypsin with alpha2M was demonstrated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis after reduction of the alpha2M-trypsin complex in urea. The amino acid composition, carbohydrate content, absorption coefficient at 280 nm, the specific refractive increment and the sedimentation coefficient for these alpha2M preparations were measured. The stability of the trypsin-binding activity of the alpha2M preparations was also studied under several storage situations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Roark D. E., Yphantis D. A. Improved ultracentrifuge cells for high-speed sedimentation equilibrium studies with interference optics. Anal Biochem. 1970 Mar;34:237–261. doi: 10.1016/0003-2697(70)90103-x. [DOI] [PubMed] [Google Scholar]
- Aune K. C., Timasheff S. N. Dimerization of alpha-chymotrypsin. I. pH dependence in the acid region. Biochemistry. 1971 Apr 27;10(9):1609–1617. doi: 10.1021/bi00785a017. [DOI] [PubMed] [Google Scholar]
- Babul J., Stellwagen E. Measurement of protein concentration with interferences optics. Anal Biochem. 1969 Apr 4;28(1):216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Dunn J. T., Spiro R. G. The alpha 2-macroglobulin of human plasma. I. Isolation and composition. J Biol Chem. 1967 Dec 10;242(23):5549–5555. [PubMed] [Google Scholar]
- Edelstein S. J., Schachman H. K. The simultaneous determination of partial specific volumes and molecular weights with microgram quantities. J Biol Chem. 1967 Jan 25;242(2):306–311. [PubMed] [Google Scholar]
- Frénoy J. P., Bourrillon R., Lippoldt R., Edelhoch H. Stability and subunit structure of human alpha2-macroglobulin. J Biol Chem. 1977 Feb 25;252(4):1129–1133. [PubMed] [Google Scholar]
- Frénoy J. P., Razafimahaleo E., Bourrillon R. Etudes sur la structure de L'a 2 -macroglobuline humaine. 3. Isolement et caractérisation d'une sous-unité. Biochim Biophys Acta. 1972 Jan 26;257(1):111–121. [PubMed] [Google Scholar]
- GOOD T. A., BESSMAN S. P. DETERMINATION OF GLUCOSAMINE AND GALACTOSAMINE USING BORATE BUFFERS FOR MODIFICATION OF THE ELSON-MORGAN AND MORGAN-ELSON REACTIONS. Anal Biochem. 1964 Nov;9:253–262. doi: 10.1016/0003-2697(64)90183-6. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Determination of alpha-2-macroglobulin as trypsin-protein esterase. Clin Chim Acta. 1966 Oct;14(4):493–501. doi: 10.1016/0009-8981(66)90037-4. [DOI] [PubMed] [Google Scholar]
- HEIMBURGER N., HEIDE K., HAUPT H., SCHULTZE H. E. BAUSTEINANALYSEN VON HUMANSERUMPROTEINEN. Clin Chim Acta. 1964 Oct;10:293–307. doi: 10.1016/0009-8981(64)90059-2. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Creeth J. M., Kekwick R. A. Thio reduction of human 2 -macroglobulin. The subunit structure. Biochem J. 1972 Mar;127(1):187–197. doi: 10.1042/bj1270187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Mihaesco C. Affinity chromatography: specific binding of hemoglobin on agarose linked haptoglobin. Biochem Biophys Res Commun. 1973 Jun 8;52(3):774–778. doi: 10.1016/0006-291x(73)91004-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEHL J. W., O'CONNELL W., DEGROOT J. MACROGLOBULIN FROM HUMAN PLASMA WHICH FORMS AN ENZYMATICALLY ACTIVE COMPOUND WITH TRYPSIN. Science. 1964 Aug 21;145(3634):821–822. doi: 10.1126/science.145.3634.821. [DOI] [PubMed] [Google Scholar]
- MILSTEIN S. W., DRISCOLL L. H. Oxidation of albumin-bound palmitate-1-C14 by adipose and hepatic tissues of the rat. J Biol Chem. 1959 Jan;234(1):19–21. [PubMed] [Google Scholar]
- Oelshlegel F. J., Jr, Schroeder J. R., Stahmann M. A. A simple procedure for basic hydrolysis of proteins and rapid determination of tryptophan using a starch column. Anal Biochem. 1970 Apr;34(2):331–337. doi: 10.1016/0003-2697(70)90116-8. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem J. 1973 Dec;135(4):867–873. doi: 10.1042/bj1350867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- RAYMOND S., WANG Y. J. Preparation and properties of acrylamide gel for use in electrophoresis. Anal Biochem. 1960 Dec 10;1:391–396. doi: 10.1016/0003-2697(60)90036-1. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SCHONENBERGER M., SCHMIDTBERGER R., SCHULTZE H. E. Uber das alpha 2-Makroglobulin. Z Naturforsch B. 1958 Dec;13B(12):761–772. [PubMed] [Google Scholar]
- Saunders R., Dyce B. J., Vannier W. E., Haverback B. J. The separation of alpha-2 macroglobulin into five components with differing electrophoretic and enzyme-binding properties. J Clin Invest. 1971 Nov;50(11):2376–2383. doi: 10.1172/JCI106736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Pannell R. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 1973 Nov 23;49(1):49–52. doi: 10.1016/0009-8981(73)90341-0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zais D. P., Roberts R. C. System for simplified discontinuous-gradient polyacrylamide-gel electrophoresis. Clin Chem. 1977 Mar;23(3):590–592. [PubMed] [Google Scholar]






