Abstract
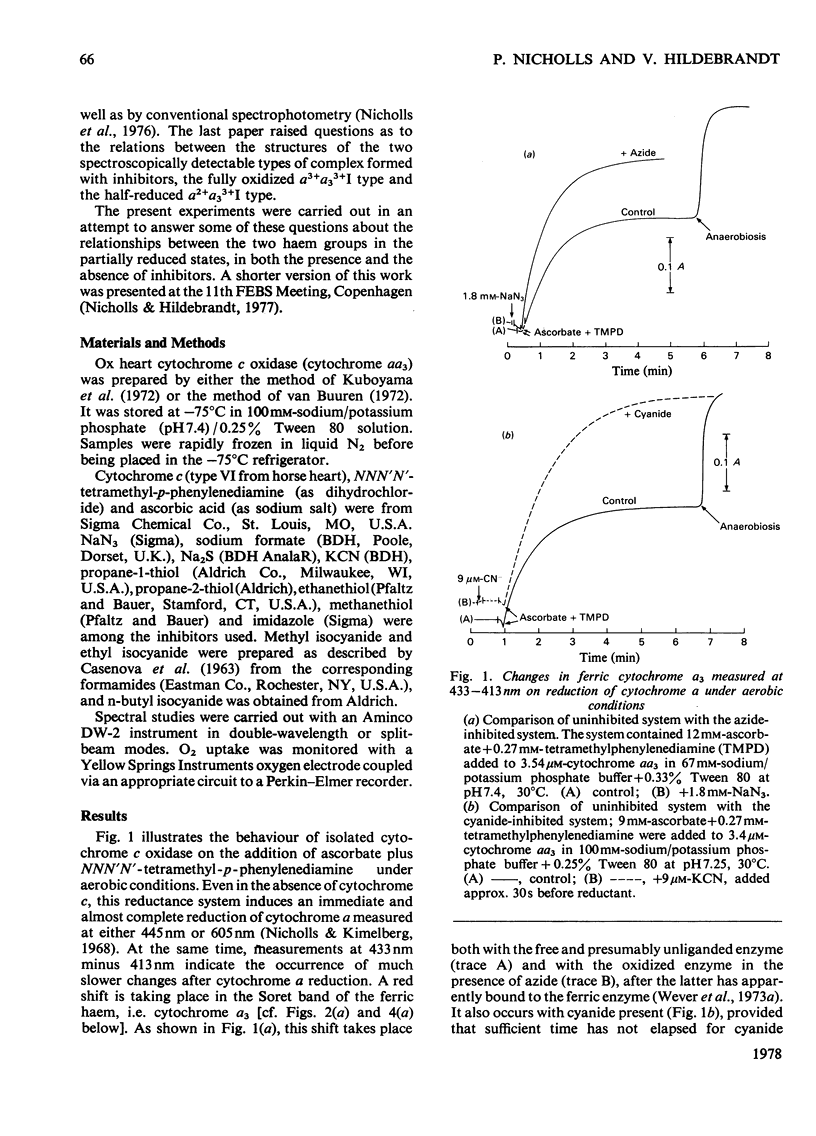
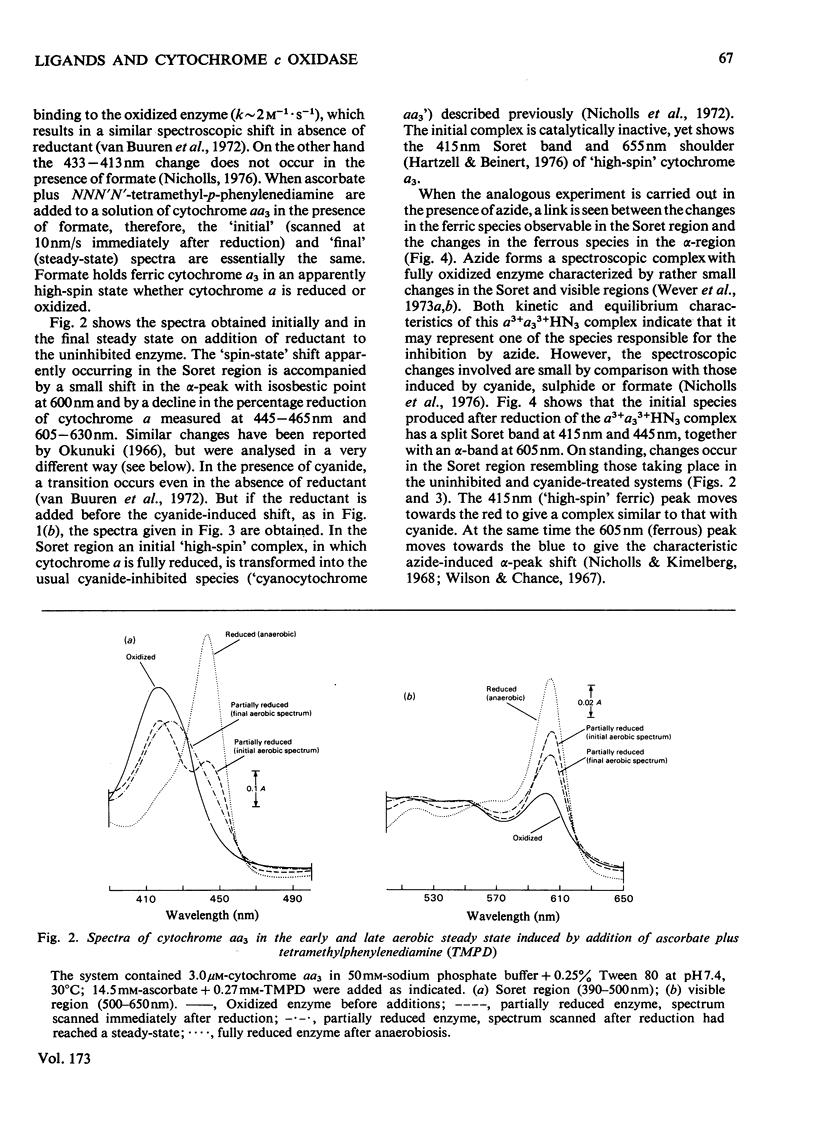
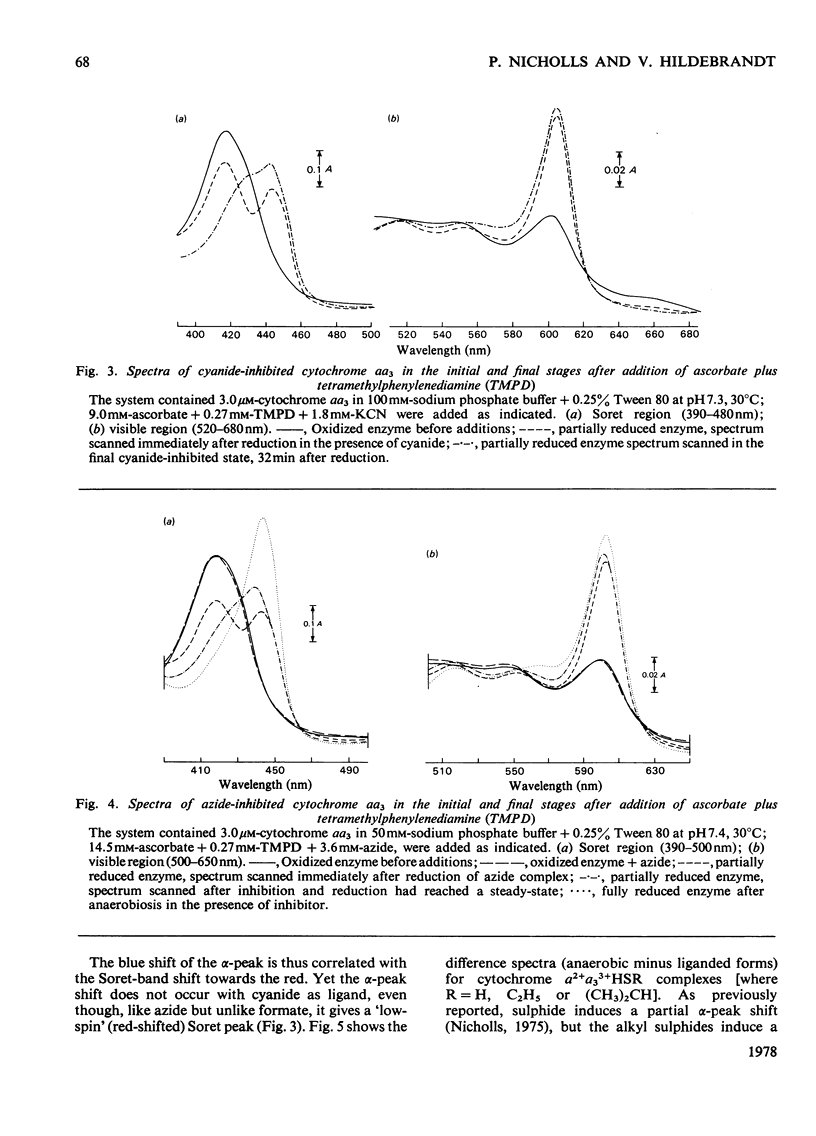
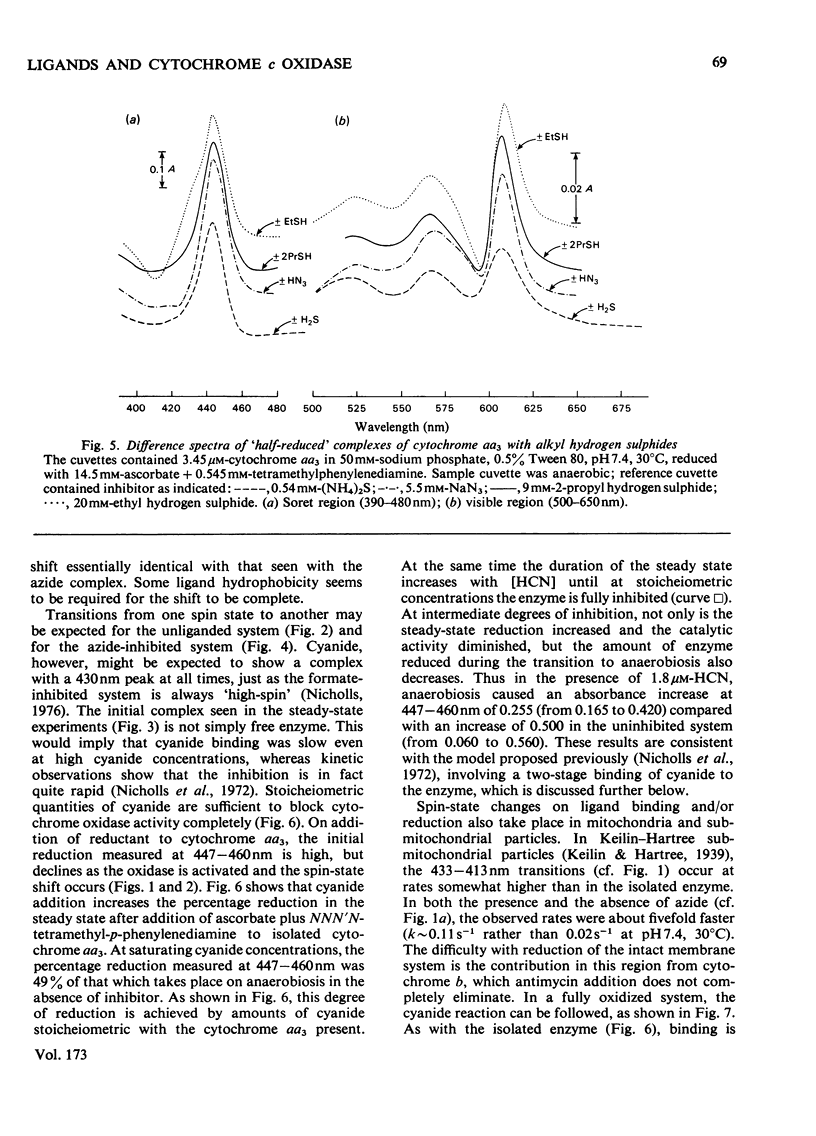
1. On addition of reductant (ascorbate plus NNN′N′-tetramethyl-p-phenylenediamine) to isolated cytochrome c oxidase (ox heart cytochrome aa3), in the presence of the inhibitors azide or cyanide, an initial partially reduced species is formed with absorption peaks at 415nm, 445nm and 605nm, which slowly gives rise to the final `half-reduced' species in whose spectrum the 415nm peak has disappeared and a new absorption is seen at 430–435nm. 2. In the absence of reductant, cyanide forms an initial complex with the enzyme with a spectrum similar to that of the uncombined form, which slowly changes into the `low-spin' cyanide form with a peak at 432nm. Azide, in absence of reductant, shifts the Soret peak slightly, but the resulting complex, which is probably thermally `mixed-spin', undergoes no further changes. 3. The Soret-peak shift of oxidized cytochrome a3 which occurs on reduction of the enzyme in the presence of azide is accompanied by a concurrent blue shift of the ferrous cytochrome a peak from 605nm to 603nm. A partial blue shift of the α-peak occurs in the half-reduced sulphide-inhibited enzyme, and a complete blue shift is seen in the analogous complexes with alkyl sulphides [a2+a33+HSR compounds, where R=CH3, C2H5 or (CH3)2CH]. 4. Analogous, albeit less readily decipherable, spectroscopic effects with the ligands imidazole and alkyl isocyanides suggest that on reduction of cytochrome a an interaction occurs between the two haem groups involving (i) a high- to low-spin change in cytochrome a3, and after this, (ii) a change in the molecular environment of the cytochrome a. The latter effect, possibly a decrease in the hydrophobicity of the haem pocket, requires that the ligands on cytochrome a3 have a bulky and partially hydrophobic character.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Brittain T., Greenwood C. Kinetic studies on the binding of cyanide to oxygenated cytochrome c oxidase. Biochem J. 1976 May 1;155(2):453–455. doi: 10.1042/bj1550453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Sato N., Nicholls P. The energy dependence of the chemical properties of cytochrome c oxidase. Arch Biochem Biophys. 1972 Jul;151(1):188–193. doi: 10.1016/0003-9861(72)90487-0. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Vänngård T., Angström J. Heme spin-states of cytochrome c oxidase derived from room temperature magnetic susceptibility measurements. FEBS Lett. 1977 Mar 15;75(1):23–27. doi: 10.1016/0014-5793(77)80044-6. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Oxido-reductive titrations of cytochrome c oxidase followed by EPR spectroscopy. Biochim Biophys Acta. 1976 Feb 16;423(2):323–338. doi: 10.1016/0005-2728(76)90189-4. [DOI] [PubMed] [Google Scholar]
- Kuboyama M., Yong F. C., King T. E. Studies on cytochrome oxidase. 8. Preparation and some properties of cardiac cytochrome oxidase. J Biol Chem. 1972 Oct 25;247(20):6375–6383. [PubMed] [Google Scholar]
- Nicholls P., Kimelberg H. K. Cytochromes a and a3. Catalytic activity and spectral shifts in situ and in solution. Biochim Biophys Acta. 1968 Jul 16;162(1):11–21. doi: 10.1016/0005-2728(68)90209-0. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Petersen L. C., Miller M., Hansen F. B. Ligand-induced spectral changes in cytochrome c oxidase and their possible significance. Biochim Biophys Acta. 1976 Nov 9;449(2):188–196. doi: 10.1016/0005-2728(76)90132-8. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976 Apr 9;430(1):13–29. doi: 10.1016/0005-2728(76)90218-8. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts of the reduced alpha-peak. Biochim Biophys Acta. 1975 Jul 8;396(1):24–35. doi: 10.1016/0005-2728(75)90186-3. [DOI] [PubMed] [Google Scholar]
- Nicholls P., van Buuren K. J., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . 8. Effect of cyanide on the catalytic activity. Biochim Biophys Acta. 1972 Sep 20;275(3):279–287. doi: 10.1016/0005-2728(72)90208-3. [DOI] [PubMed] [Google Scholar]
- Palmer G., Babcock G. T., Vickery L. E. A model for cytochrome oxidase. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2206–2210. doi: 10.1073/pnas.73.7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. Determination of the heme spin states in cytochrome c oxidase using magnetic circular dichroism. FEBS Lett. 1976 Aug 1;67(1):94–98. doi: 10.1016/0014-5793(76)80877-0. [DOI] [PubMed] [Google Scholar]
- Tsudzuki T., Okunuki K. EPR studies on cytochrome oxidase at 20 degree K. J Biochem. 1969 Aug;66(2):281–283. doi: 10.1093/oxfordjournals.jbchem.a129145. [DOI] [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- Wever R., Muijsers A. O., van Gelder B. F., Bakker E. P., van Buuren K. J. Biochemical and biophysical studies on cytochrome c oxidase. XI. Reaction with azide. Biochim Biophys Acta. 1973 Oct 19;325(1):1–7. doi: 10.1016/0005-2728(73)90144-8. [DOI] [PubMed] [Google Scholar]
- Wever R., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. XII. Kinetics of azide binding. Biochim Biophys Acta. 1973 Oct 19;325(1):8–15. doi: 10.1016/0005-2728(73)90145-x. [DOI] [PubMed] [Google Scholar]
- Wever R., van GELDER B. F., Dervartanian D. V. Biochemical and biophysical studies on cytochrome c oxidase. XX. Reaction with sulphide. Biochim Biophys Acta. 1975 May 15;387(2):189–193. doi: 10.1016/0005-2728(75)90102-4. [DOI] [PubMed] [Google Scholar]
- Wikström K. F., Harmon H. J., Ingledew W. J., Chance B. A re-evaluation of the spectral, potentiometric and energy-linked properties of cytochrome c oxidase in mitochondria. FEBS Lett. 1976 Jun 15;65(3):259–277. doi: 10.1016/0014-5793(76)80127-5. [DOI] [PubMed] [Google Scholar]
- Wikström M. K., Saari H. T. Conformational changes in cytochrome aa3 and ATP synthetase of the mitochondrial membrane and their role in mitochondrial energy transduction. Mol Cell Biochem. 1976 Mar 26;11(1):17–33. doi: 10.1007/BF01792831. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Chance B. Azide inhibition of mitochondrial electron transport. I. The aerobic steady state of succinate oxidation. Biochim Biophys Acta. 1967 May 9;131(3):421–430. doi: 10.1016/0005-2728(67)90002-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Orii Y. Location of heme a in cytochrome a. I. Combination of alkyl isonitriles with cytochrome a. J Biochem. 1973 May;73(5):1049–1059. doi: 10.1093/oxfordjournals.jbchem.a130159. [DOI] [PubMed] [Google Scholar]
- Yong F. C., King T. E. Studies on cytochrome oxidase. IX. Heme-copper interaction. J Biol Chem. 1972 Oct 25;247(20):6384–6388. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]


