Abstract
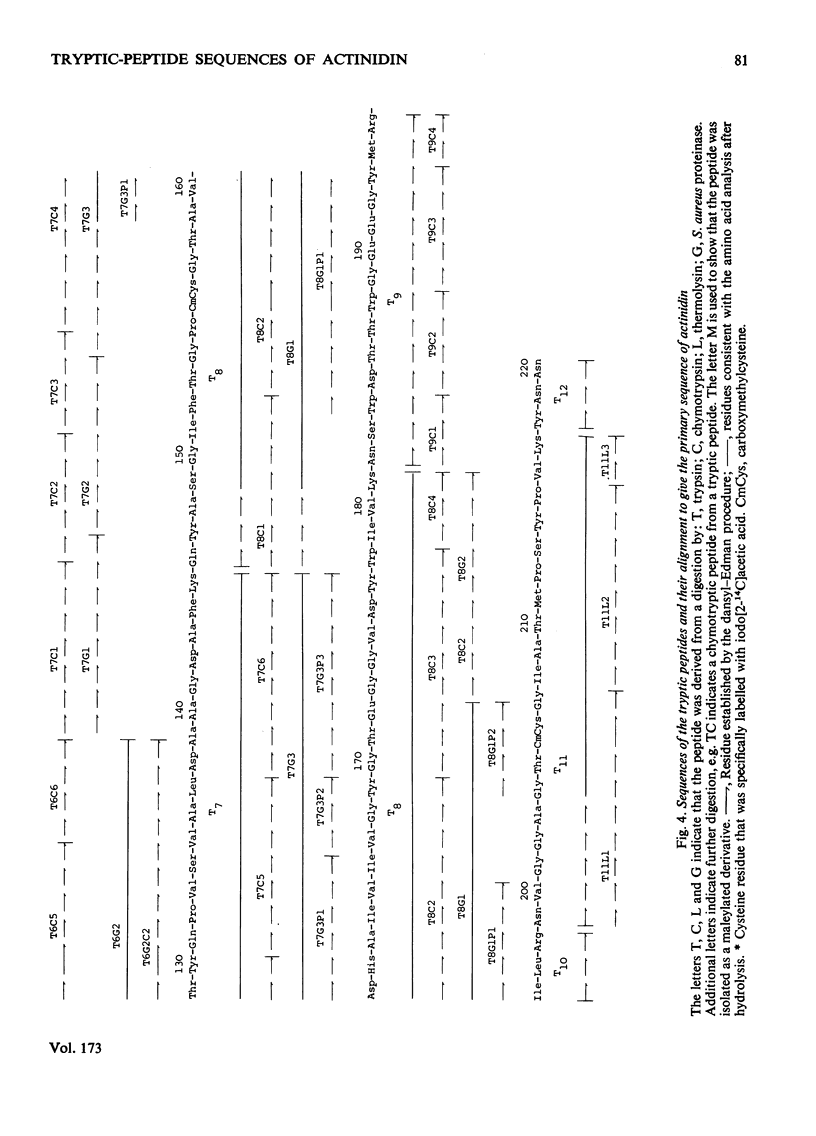
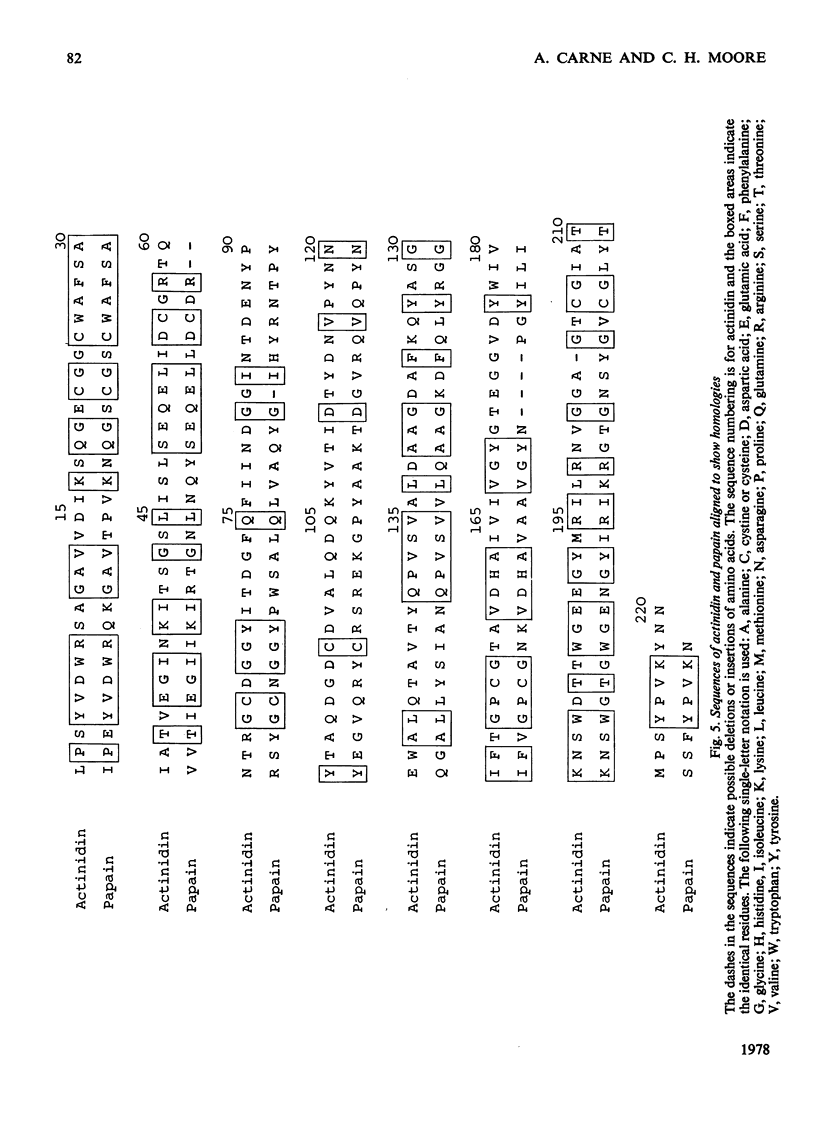
The amino acid sequences of the tryptic peptides of the thiol proteinase actinidin from Actinidia chinensis were determined by the manual dansyl--Edman procedure. There are 12 tryptic peptides, which give a polypeptide chain of 220 residues with a mol.wt. of 23500. An alignment of the tryptic peptides was made by using the X-ray-crystallographic data of Baker [(1977) J. Mol. Biol. 115, 263--277] determined at 0.28 nm resolution on crystalline actinidin. Detailed evidence for the amino acid sequences of the tryptic peptides has been deposited as Supplementary Publication SUP 50083 (14 pages) at the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978) 169, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCUS A. C. Proteolytic enzyme of Actinidia chinensis. Biochim Biophys Acta. 1959 May;33(1):242–244. doi: 10.1016/0006-3002(59)90522-0. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Preliminary crystallographic data for actinidin, a thiol protease from Actinidia chinensis. J Mol Biol. 1973 Mar 5;74(3):411–412. doi: 10.1016/0022-2836(73)90382-3. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution. J Mol Biol. 1977 Sep 25;115(3):263–277. doi: 10.1016/0022-2836(77)90154-1. [DOI] [PubMed] [Google Scholar]
- Baker E. N. The structure of actinidin at 5-5 A resolution. J Mol Biol. 1976 Feb 25;101(2):185–196. doi: 10.1016/0022-2836(76)90371-5. [DOI] [PubMed] [Google Scholar]
- Boland M. J., Hardman M. J. Kinetic studies on the thiol protease from Actinidia chinensis. FEBS Lett. 1972 Nov 1;27(2):282–284. doi: 10.1016/0014-5793(72)80641-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drenth J., Hol W. G., Visser J. W., Sluyterman L. A. Papain in water-rich and in methanol-rich media; crystallization and conformation. J Mol Biol. 1968 Jul 14;34(2):369–371. doi: 10.1016/0022-2836(68)90262-3. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Emmens M., Welling G. W., Beintema J. J. The amino acid sequence of pike-whale (lesser-rorqual) pancreatic ribonuclease. Biochem J. 1976 Aug 1;157(2):317–323. doi: 10.1042/bj1570317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK H., PETERSEN H. Eine Methode zur quantitativen Bestimmung von Histidin in Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1955;299(1):1–5. [PubMed] [Google Scholar]
- Goto K., Murachi T., Takahashi N. Structural studies on stem bromelain isolation, characterization and alignment of the cyanogen bromide fragments. FEBS Lett. 1976 Feb 1;62(1):93–95. doi: 10.1016/0014-5793(76)80024-5. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Haworth C. An improved technique for the analysis of amino acids and related compounds on thin layers of cellulose. II. The quantitative determination of amino acids in protein hydrolysates. J Chromatogr. 1969 Aug 5;43(1):84–92. doi: 10.1016/s0021-9673(00)99169-6. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Completion of the amino acid sequence of papain. Biochem J. 1969 Sep;114(2):279–288. doi: 10.1042/bj1140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues of stem bromelain. Biochem J. 1970 Apr;117(2):341–346. doi: 10.1042/bj1170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues, and the buried cysteine residue in ficin. Biochem J. 1970 Apr;117(2):333–340. doi: 10.1042/bj1170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHT A., FRATER R., KIMMEL J. R., SMITH E. L. CURRENT STATUS OF THE STRUCTURE OF PAPAIN: THE LINEAR SEQUENCE, ACTIVE SULFHYDRYL GROUP, AND THE DISULFIDE BRIDGES. Proc Natl Acad Sci U S A. 1964 Nov;52:1276–1283. doi: 10.1073/pnas.52.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall M. A. Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme. Eur J Biochem. 1970 Jun;14(2):214–221. doi: 10.1111/j.1432-1033.1970.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Mitchel R. E., Chaiken I. M., Smith E. L. The complete amino acid sequence of papain. Additions and corrections. J Biol Chem. 1970 Jul 25;245(14):3485–3492. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- Scoffone E., Fontana A., Rocchi R. Sulfenyl halides as modifying reagents for polypeptides and proteins. I. Modification of tryptophan residues. Biochemistry. 1968 Mar;7(3):971–979. doi: 10.1021/bi00843a014. [DOI] [PubMed] [Google Scholar]
- Tang J., Hartley B. S. A diagonal electrophoretic method for selective purification of methionine peptides. Biochem J. 1967 Feb;102(2):593–599. doi: 10.1042/bj1020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]



