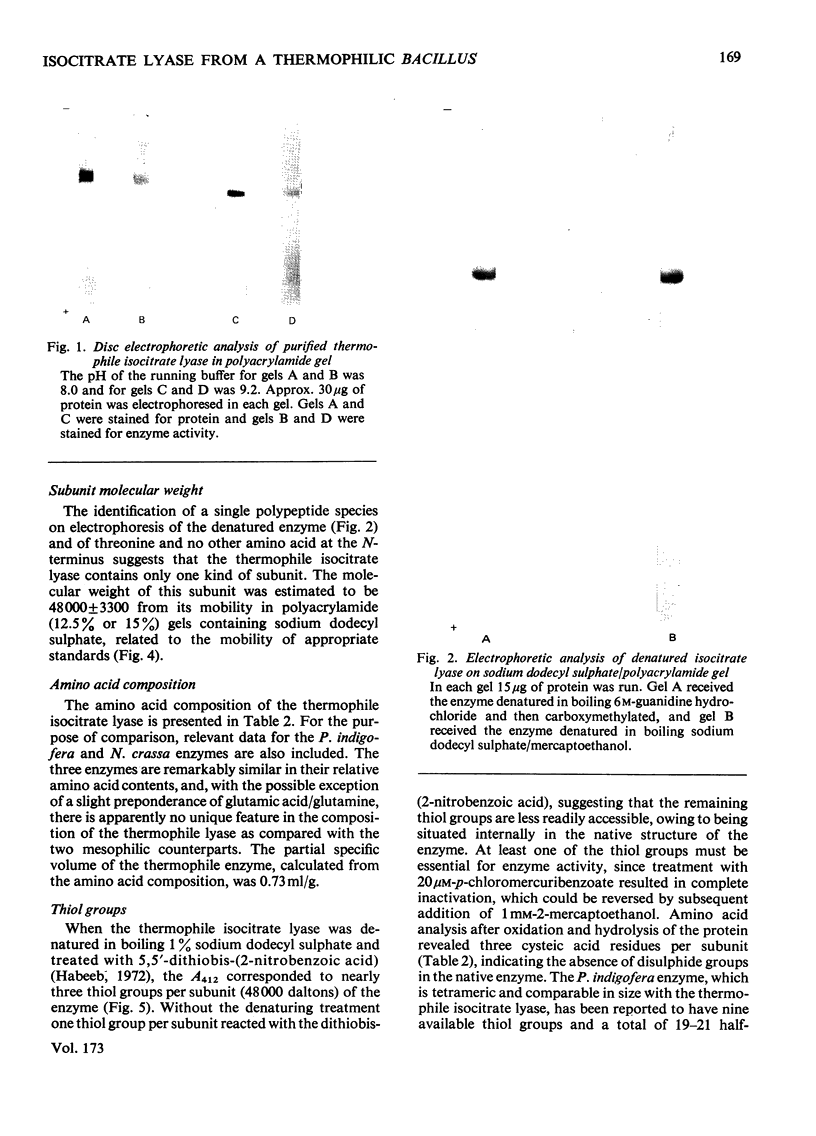
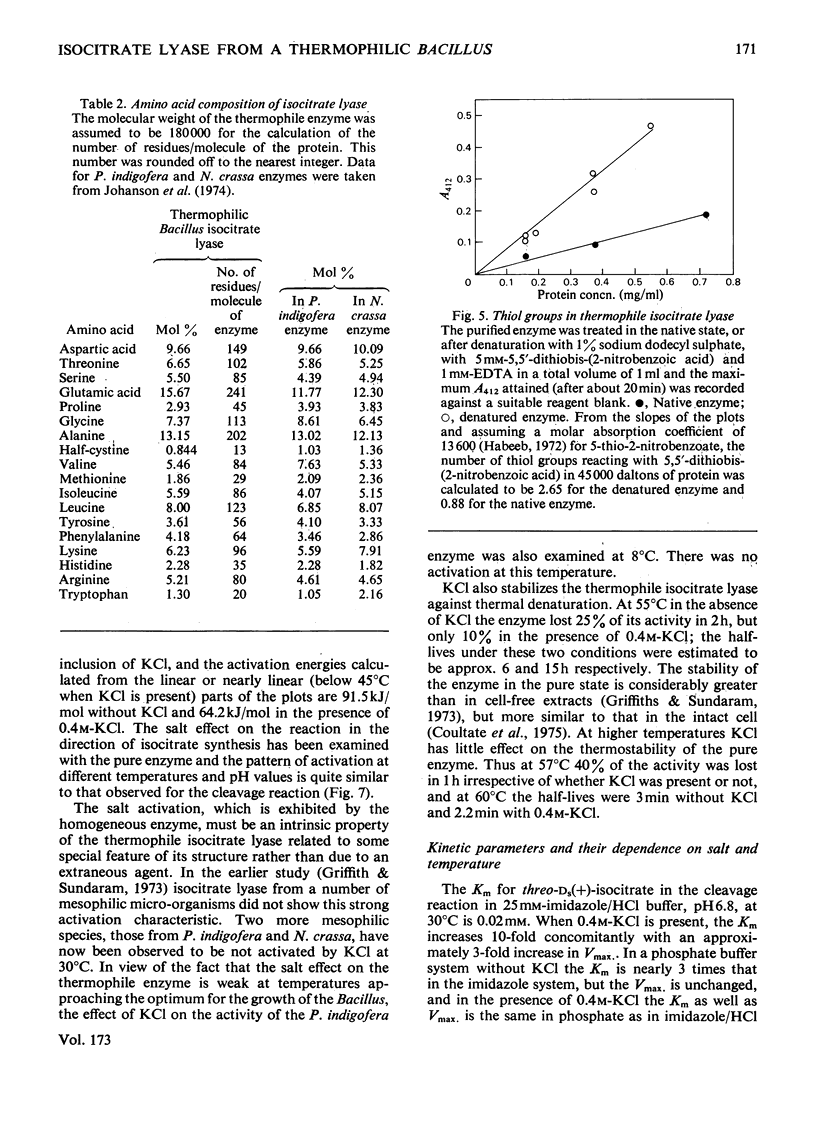
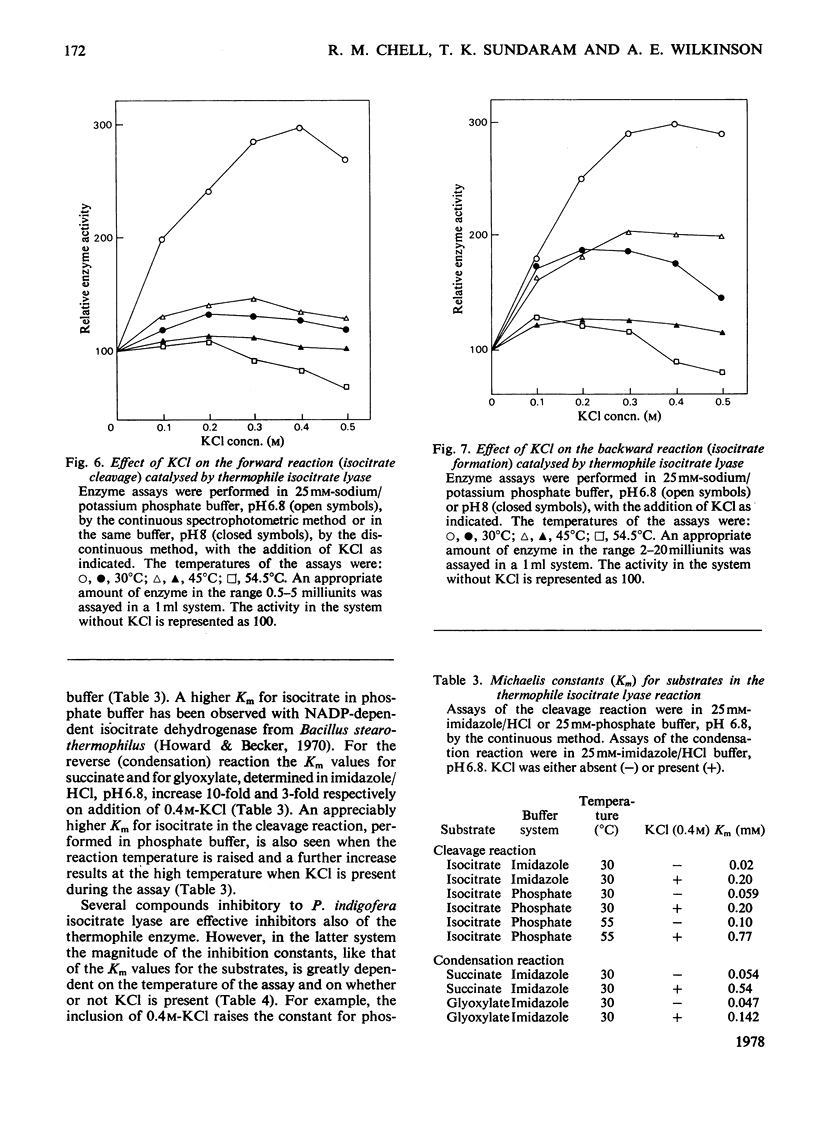
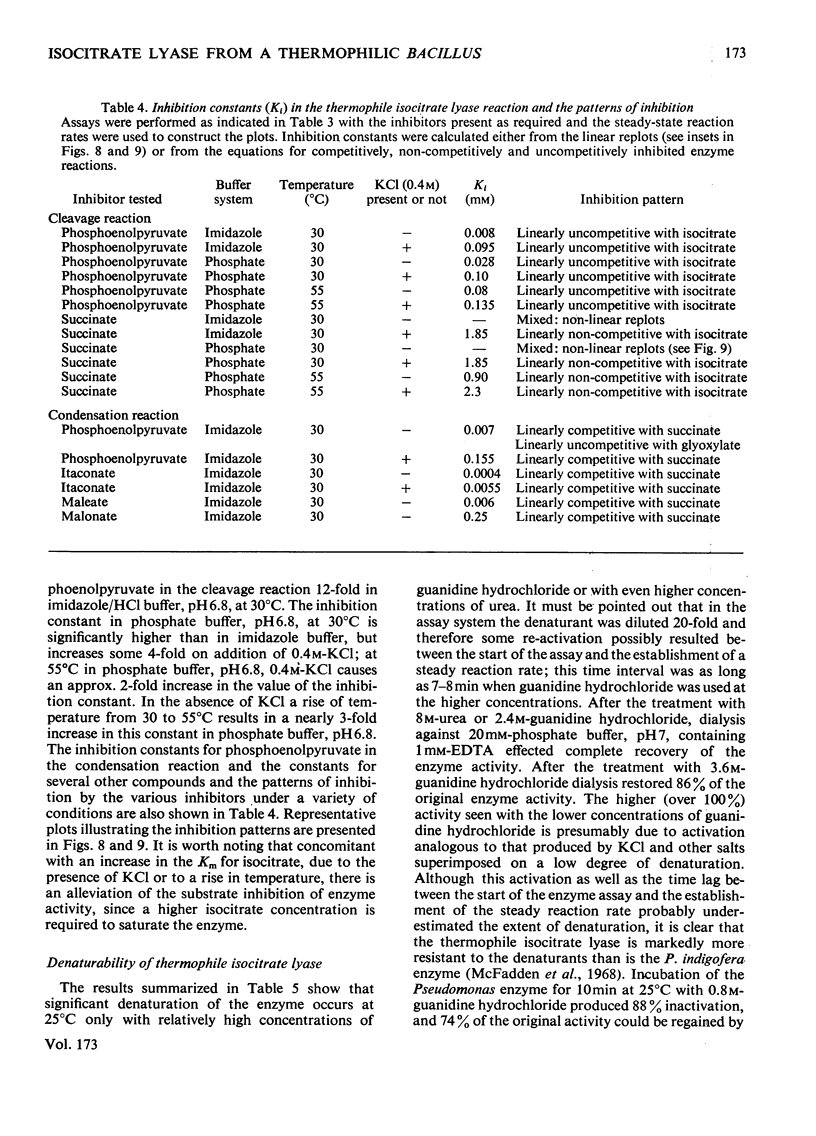
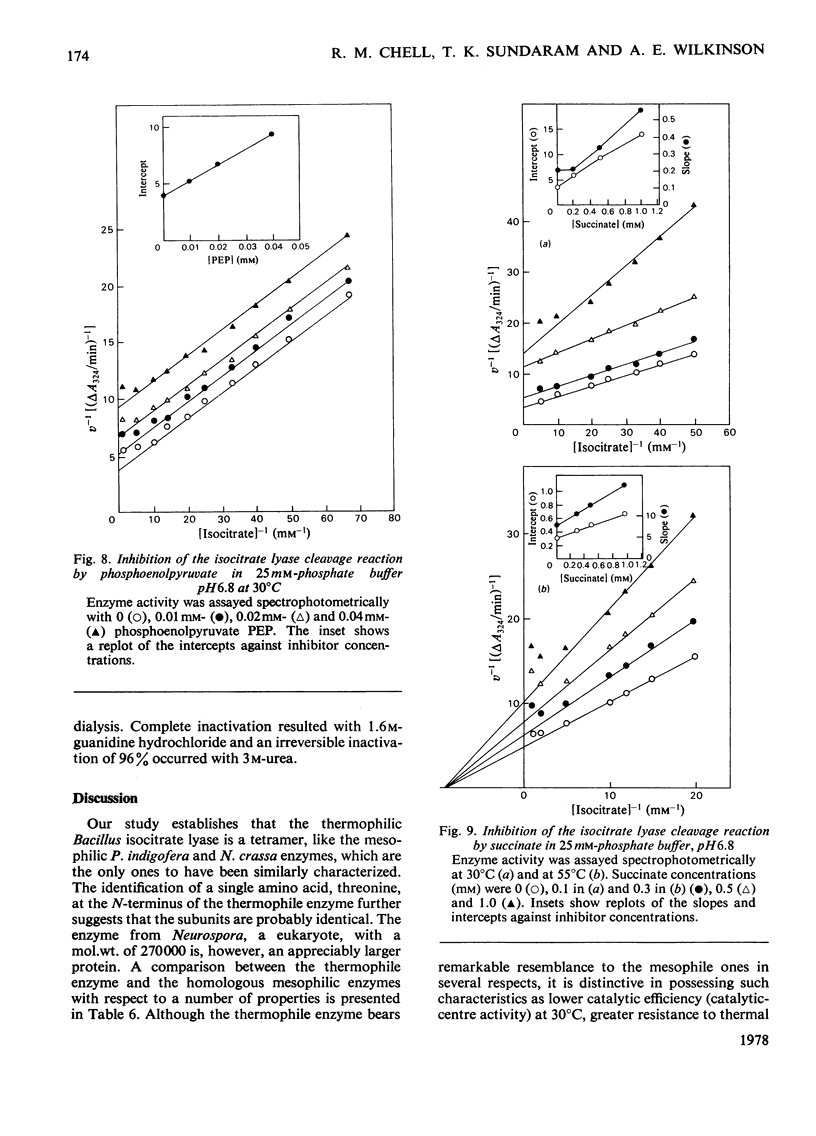
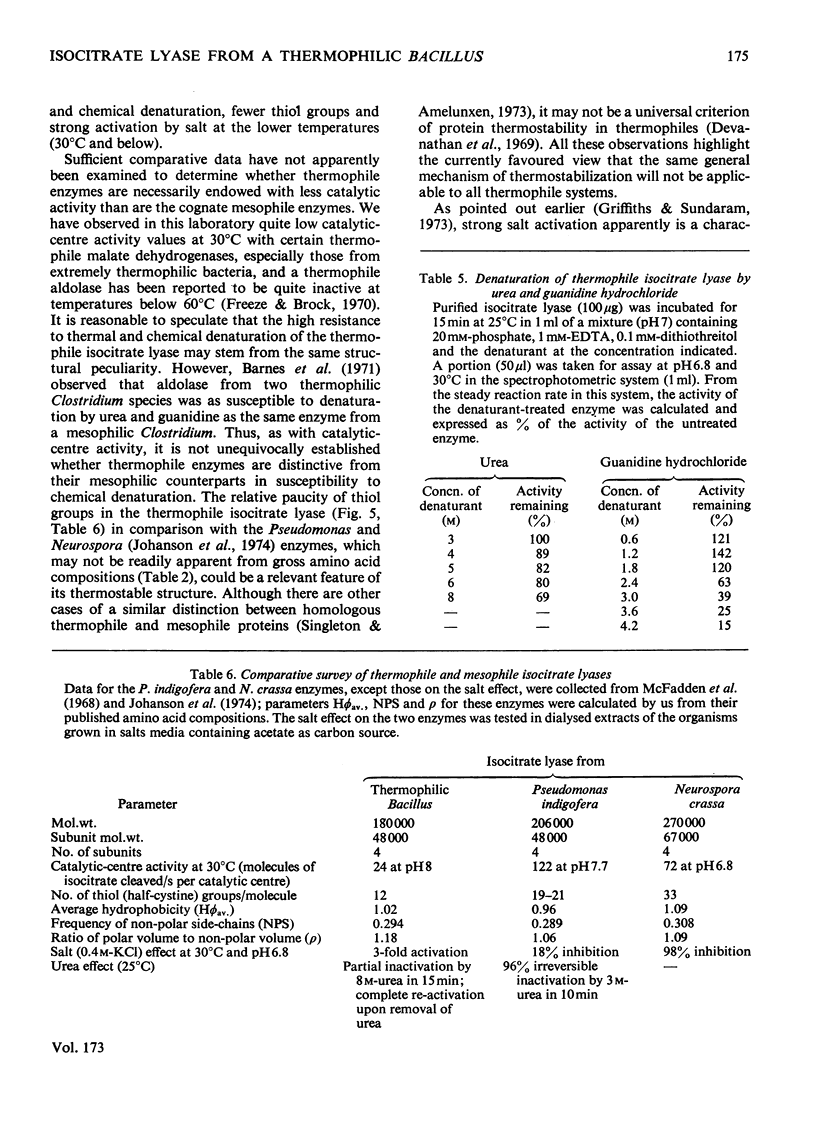
Abstract
Isocitrate lyase was isolated in homogeneous state from a thermophilic Bacillus. The enzyme has a mol.wt. of 180000 and a pI of 4.5 and contains threonine as the N-terminal residue. It resembles in size the cognate enzyme from the mesophilic bacterium Pseudomonas indigofera, but is smaller than the enzyme from the eukaryotic fungus Neurospora crassa. All three lyases are tetramers and similar in amino acid composition, but the thermophile enzyme is distinctive from its mesophilic coutnerparts in possessing a lower catalytic-centre activity, greater resistance to chemical and thermal denaturation and fewer thiol groups and in being strongly activated by salts. Salt activation, by 0.4M-KCl, is about 3-fold at 30 degrees C and pH 6.8 and weakens progressively as the temperature or pH is raised. The activation is probably due to a change in the enzyme conformation caused by the electrolyte modifying the interaction between charged groups or between hydrophobic groups in protein. The possible significance of the salt activation, of the relative paucity of thiol groups and of the greater resistance to chemical denaturants is discussed. Besides its effect on the Vmax., KCl produces large increases in the magnitude of several kinetic parameters. A rise in reaction temperature from 30 to 55 degrees C produces a somewhat similar result. In view of these peculiar features, the patterns of inhibition of enzyme activity by compounds such as succinate and phosphoenolpyruvate were examined at 30 and 55 degrees C in the presence and absence of KCl.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Akagi J. M., Himes R. H. Properties of fructose-1,6-diphosphate aldolase from two thermophilic and a mesophilic clostridia. Biochim Biophys Acta. 1971 Jan 13;227(1):199–203. doi: 10.1016/0005-2744(71)90180-x. [DOI] [PubMed] [Google Scholar]
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J., Sundaram T. K., Kornberg H. L. Properties and regulation of pyruvate carboxylase from Bacillus stearothermophilus. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):1–19. doi: 10.1098/rspb.1970.0030. [DOI] [PubMed] [Google Scholar]
- Chell R. M., Sundaram T. K. Mutants of Bacillus stearothermophilus de-repressed for isocitrate lyase and malate synthase. Biochem Soc Trans. 1975;3(2):306–309. doi: 10.1042/bst0030306. [DOI] [PubMed] [Google Scholar]
- Coultate T. P., Sundaram T. K., Cazzulo J. J. Stability of protein and ribonucleic acid in Bacillus stearothermophilus. J Gen Microbiol. 1975 Dec;91(2):383–390. doi: 10.1099/00221287-91-2-383. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Pain R. H. The determination of molecular weights of biological macromolecules by ultracentrifuge methods. Prog Biophys Mol Biol. 1967;17:217–287. doi: 10.1016/0079-6107(67)90008-9. [DOI] [PubMed] [Google Scholar]
- Daron H. H. Occurrence of isocitrate lyase in a thermophilic bacillus species. J Bacteriol. 1967 Feb;93(2):703–710. doi: 10.1128/jb.93.2.703-710.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenathan T., Akagi J. M., Hersh R. T., Himes R. H. Ferredoxin from two thermophilic clostridia. J Biol Chem. 1969 Jun 10;244(11):2846–2853. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Epstein I., Grossowicz N. Prototrophic thermophilic bacillus: isolation, properties, and kinetics of growth. J Bacteriol. 1969 Aug;99(2):414–417. doi: 10.1128/jb.99.2.414-417.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER H. F. A LIMITING LAW RELATING THE SIZE AND SHAPE OF PROTEIN MOLECULES TO THEIR COMPOSITION. Proc Natl Acad Sci U S A. 1964 Jun;51:1285–1291. doi: 10.1073/pnas.51.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H., Brock T. D. Thermostable aldolase from Thermus aquaticus. J Bacteriol. 1970 Feb;101(2):541–550. doi: 10.1128/jb.101.2.541-550.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. W., Sundaram T. K. Isocitrate lyase from a thermophilic Bacillus: effect of salts on enzyme activity. J Bacteriol. 1973 Dec;116(3):1160–1169. doi: 10.1128/jb.116.3.1160-1169.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. L., Becker R. R. Isolation and some properties of the triphosphopyridine nucleotide isocitrate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1970 Jun;245(12):3186–3194. [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. II. Composition, quaternary structure, C-terminus, and active-site modification. Biochim Biophys Acta. 1974 Oct 17;364(2):341–352. doi: 10.1016/0005-2744(74)90019-9. [DOI] [PubMed] [Google Scholar]
- John P. C., Syrett P. J. The purification and properties of isocitrate lyase from Chlorella. Biochem J. 1967 Oct;105(1):409–416. doi: 10.1042/bj1050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Rao G. R., Cohen A. L., Roche T. E. Isocitrate lyase from Pseudomonas indigofera. V. Subunits and terminal residues and the relation to catalytic activity. Biochemistry. 1968 Oct;7(10):3574–3582. doi: 10.1021/bi00850a035. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Reiss U., Rothstein M. Isocitrate lyase from the free-living nematode, Tubatrix aceti: purification and properties. Biochemistry. 1974 Apr 23;13(9):1796–1800. doi: 10.1021/bi00706a003. [DOI] [PubMed] [Google Scholar]
- SHIIO I., SHIIO T., MCFADDEN B. A. ISOCITRATE LYASE FROM PSEUDOMONAS INDIGOFERA. I. PREPARATION, AMINO ACID COMPOSITION AND MOLECULAR WEIGHT. Biochim Biophys Acta. 1965 Jan;96:114–122. doi: 10.1016/0005-2787(65)90615-5. [DOI] [PubMed] [Google Scholar]
- Shiio I., McFadden B. A. Isocitrate lyase from Pseudomonas indigofera. 3. Sulfhydryl groups and enzyme activity. Biochim Biophys Acta. 1965 Sep 20;105(3):496–505. doi: 10.1016/s0926-6593(65)80234-x. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram T. K., Cazzulo J. J., Kornberg H. L. Anaplerotic CO2 fixation in mesophilic and thermophilic bacilli. Biochim Biophys Acta. 1969 Nov 18;192(2):355–357. doi: 10.1016/0304-4165(69)90377-8. [DOI] [PubMed] [Google Scholar]
- Sundaram T. K. Physiological role of pyruvate carboxylase in a thermophilic bacillus. J Bacteriol. 1973 Feb;113(2):549–557. doi: 10.1128/jb.113.2.549-557.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAUGH D. F. Protein-protein interactions. Adv Protein Chem. 1954;9:325–437. doi: 10.1016/s0065-3233(08)60210-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williams J. O., Roche T. E., McFadden B. A. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971 Apr 13;10(8):1384–1390. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]