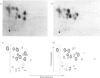
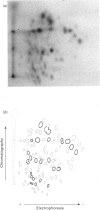
Abstract
1. The two major polypeptides (P1 and P2) of erythrocyte-membrane spectrin were isolated by preparative polyacrylamide-gel electrophoresis. 2. The two polypeptides were shown to possess similar amino acid compositions, both with the characteristically high glutamate and leucine contents of the parent spectrin. 3. The tryptic-peptide 'maps' of the two polypeptides were prepared by a combination of t.l.c. and electrophoresis. 4. Radioactive peptides were prepared by [14C]carboxymethylation and chloramine-T-catalysed [125I]iodination. 5. 'Maps' of both sets of peptides demonstrate a marked similarity between the two polypeptides. 6. These new data confirm earlier evidence for the similarity of the two chains. 7. The number of peptides in the 'maps' of carboxymethylated peptides suggest that polypeptides P1 and P2 are not aggregates.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Tanner M. J. Reducible components in the proteins of human erythrocyte membrane. Biochim Biophys Acta. 1976 May 20;434(1):51–57. doi: 10.1016/0005-2795(76)90034-9. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Bhakdi S., Bog-Hansen T. C., Knüfermann H., Wallach D. F. Quantitative immunoelectrophoresis of proteins in human erythrocyte membranes. Analysis of protein bands obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1975 Nov 3;406(4):489–504. doi: 10.1016/0005-2736(75)90027-9. [DOI] [PubMed] [Google Scholar]
- Brandon D. L. Myosin-like polypeptides in plasma membrane preparations. FEBS Lett. 1975 Oct 15;58(1):349–352. doi: 10.1016/0014-5793(75)80295-x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J., McBay W., Maddy A. H. The N-terminal heterogeneity of edta-extractable erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Mar 28;386(1):107–119. doi: 10.1016/0005-2795(75)90251-2. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Shotton D. M., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. II. The influence of spectrin aggregation. Biochim Biophys Acta. 1976 Feb 19;426(1):101–122. doi: 10.1016/0005-2736(76)90433-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Nachbar M. S., Salton M. R. Immunochemistry and peptide mapping of Micrococcus lysodeikticus membrane proteins. Biochim Biophys Acta. 1971 Jul 6;241(1):30–41. doi: 10.1016/0005-2736(71)90300-2. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Boughter J. M., Morazzani M. Evidence for multiple polypeptide chains in the membrane protein spectrin. Biochemistry. 1974 Jul 16;13(15):3036–3041. doi: 10.1021/bi00712a006. [DOI] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Guthrow C. E., Jr, Allen J. E., Rasmussen H. Phosphorylation of an endogenous membrane protein by an endogenous, membrane-associated cyclic adenosine 3',5'-monophosphate-dependent protein kinase in human erythrocyte ghosts. J Biol Chem. 1972 Dec 25;247(24):8145–8153. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Harris J. R., Maddy A. H. An electron microscopic study of some protein fractions from bovine erythrocyte ghosts. J Ultrastruct Res. 1974 Aug;48(2):190–200. doi: 10.1016/s0022-5320(74)80076-6. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Hulla F. W., Gratzer W. B. Association of high-molecular weight proteins in the red cell membrane. FEBS Lett. 1972 Sep 15;25(2):275–278. doi: 10.1016/0014-5793(72)80502-7. [DOI] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The structure of the major protein of the human erythrocyte membrane. Characterization of the intact protein and major fragments. Biochem J. 1977 Jan 1;161(1):139–147. doi: 10.1042/bj1610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirgl V. Contractile proteins from human erythrocyte membranes: some notes on extraction of an acetomyosin-like protein and some of its physical and chemical properties. Folia Biol (Praha) 1971;17(6):392–398. [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Oct;105(1):361–370. doi: 10.1042/bj1050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer B., Hicks R. M., Christodoulides L., Beale D. Studies of the chemistry of the luminal plasma membrane of rat bladder epithelial cells. Biochim Biophys Acta. 1973 Jun 22;311(2):180–190. doi: 10.1016/0005-2736(73)90265-4. [DOI] [PubMed] [Google Scholar]
- Knufermann H., Bhakdi S., Schmidt-Ullrich R., Wallach D. F. N-terminal amino acid analysis reveal peptide heterogeneity in major electrophoretic protein components of erythrocyte ghosts. Biochim Biophys Acta. 1973 Dec 22;330(3):356–361. doi: 10.1016/0005-2736(73)90246-0. [DOI] [PubMed] [Google Scholar]
- Maddy A. H. A critical evaluation of the analysis of membrane proteins by polyacrylamide gel electrophoresis in the presence of dodecyl sulphate. J Theor Biol. 1976 Oct 21;62(2):315–326. doi: 10.1016/0022-5193(76)90123-5. [DOI] [PubMed] [Google Scholar]
- Maddy A. H. The properties of the protein of the plasma membrane of ox erythrocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):193–200. doi: 10.1016/0304-4165(66)90166-8. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- OHNISHI T. Extraction of actin- and myosin-like proteins from erythrocyte membrane. J Biochem. 1962 Oct;52:307–308. doi: 10.1093/oxfordjournals.jbchem.a127620. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Tidmarsh S., Gratzer W. B. Integrity of polypeptide chains of spectrin from human erythrocytes. Arch Biochem Biophys. 1976 Feb;172(2):654–660. doi: 10.1016/0003-9861(76)90120-x. [DOI] [PubMed] [Google Scholar]
- Ralston G. B. The isolation of aggregates of spectrin from bovine erythrocyte membranes. Aust J Biol Sci. 1975 Jun;28(3):259–266. doi: 10.1071/bi9750259. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Sharp M., Reynolds J. A., Tanford C. Erythrocyte spectrin. Purification in deoxycholate and preliminary characterization. Biochemistry. 1976 May 4;15(9):1897–1904. doi: 10.1021/bi00654a016. [DOI] [PubMed] [Google Scholar]
- Shapiro D. L., Marchesi V. T. Phosphorylation in membranes of intact human erythrocytes. J Biol Chem. 1977 Jan 25;252(2):508–517. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi S. L., Marchesi V. T., Steers E., Jr A comparative study of spectrin: a protein isolated from red blood cell membranes. Biochim Biophys Acta. 1970 Jan 20;200(1):125–131. doi: 10.1016/0005-2795(70)90050-4. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]