Abstract
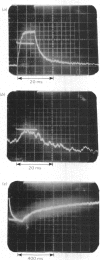
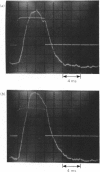
An electric field causes partial alignment of macromolecules in a dilute solution. The accompanying changes in the solution birefringence offer a sensitive and quick means of monitoring the rates of particle orientation and hence the size of the solute molecules. Such measurements are reported for dilute solutions of proteoglycans in the absence and presence of added hyaluronic acid. The proteoglycan molecules are shown to be some 580 nm long. In the presence of hyaluronic acid they form aggregates that appear to be consistent with the model previously proposed in which the proteoglycans attach radially to the extended hyaluronic acid chain. The electric-birefringence relaxation rates indicate aggregates of similar length to that of the extended hyaluronic acid chain, with the proteoglycans spaced on average at 29nm intervals. A proteoglycan sample the cystine residues of which had been reduced and alkylated showed no evidence of aggregation with hyaluronic acid up to the concentrations of the acid corresponding to 1% of the total uronic acid content. The electric-birefringence method is shown to have a large potential in the study of associating polysaccharide solutions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. L., Wang J. L. Ionic polysaccharides. 3. Dilute solution properties of hyaluronic acid fractions. Biopolymers. 1970;9(7):799–810. doi: 10.1002/bip.1970.360090706. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Lohmander S., Hjerpe A. Proteoglycans of mineralizing rib and epiphyseal cartilage. Biochim Biophys Acta. 1975 Sep 8;404(1):93–109. doi: 10.1016/0304-4165(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Pasternack S. G., Veis A., Breen M. Solvent-dependent changes in proteoglycan subunit conformation in aqueous guanidine hydrochloride solutions. J Biol Chem. 1974 Apr 10;249(7):2206–2211. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]