Abstract
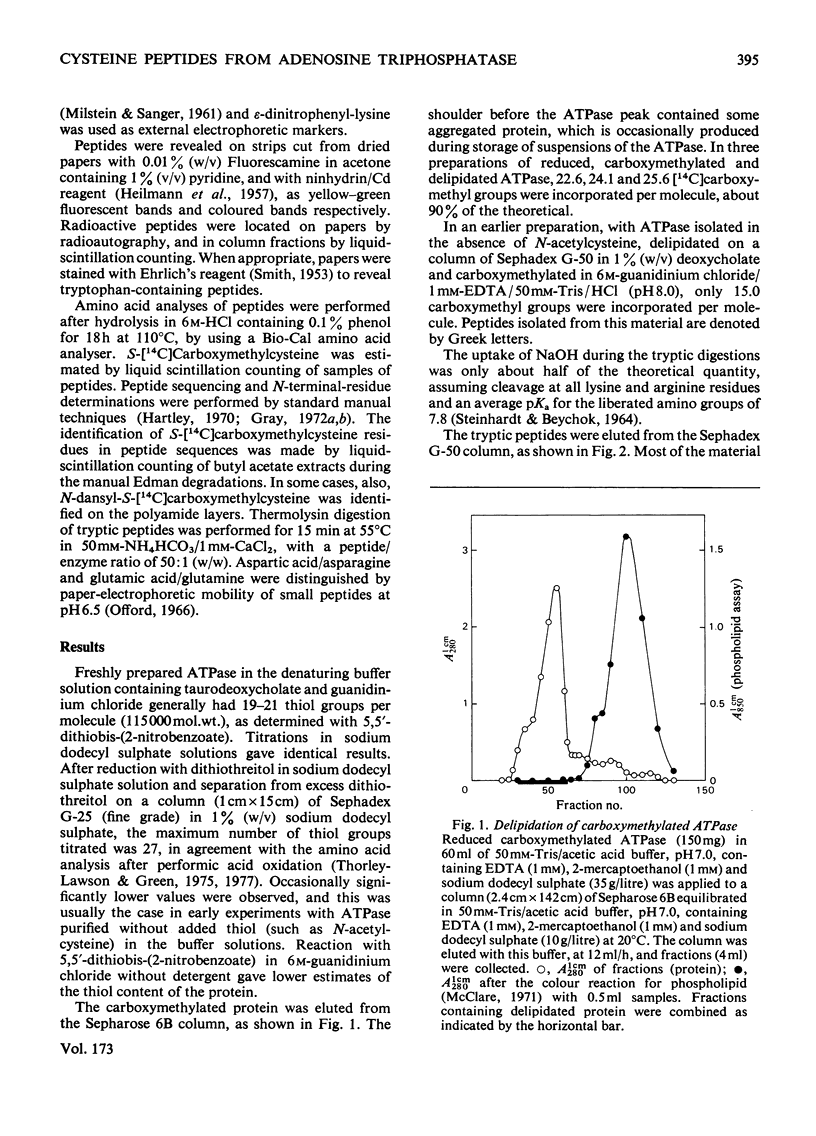
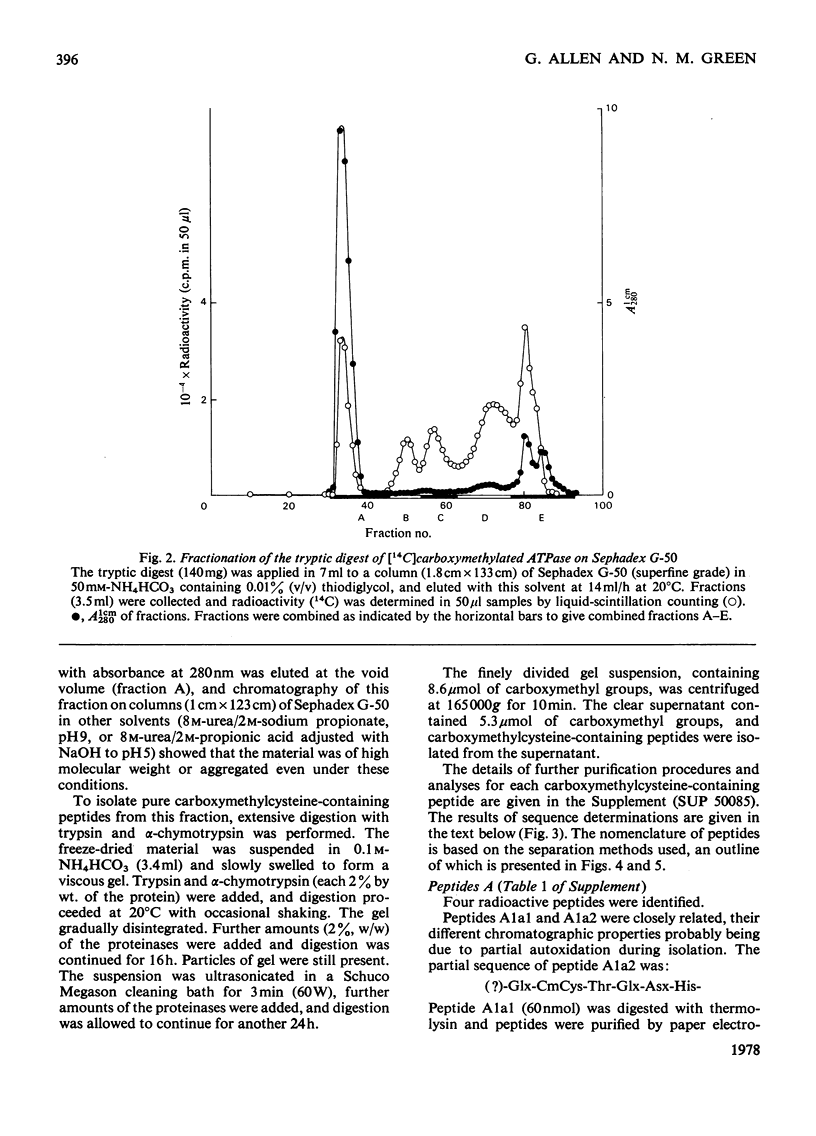
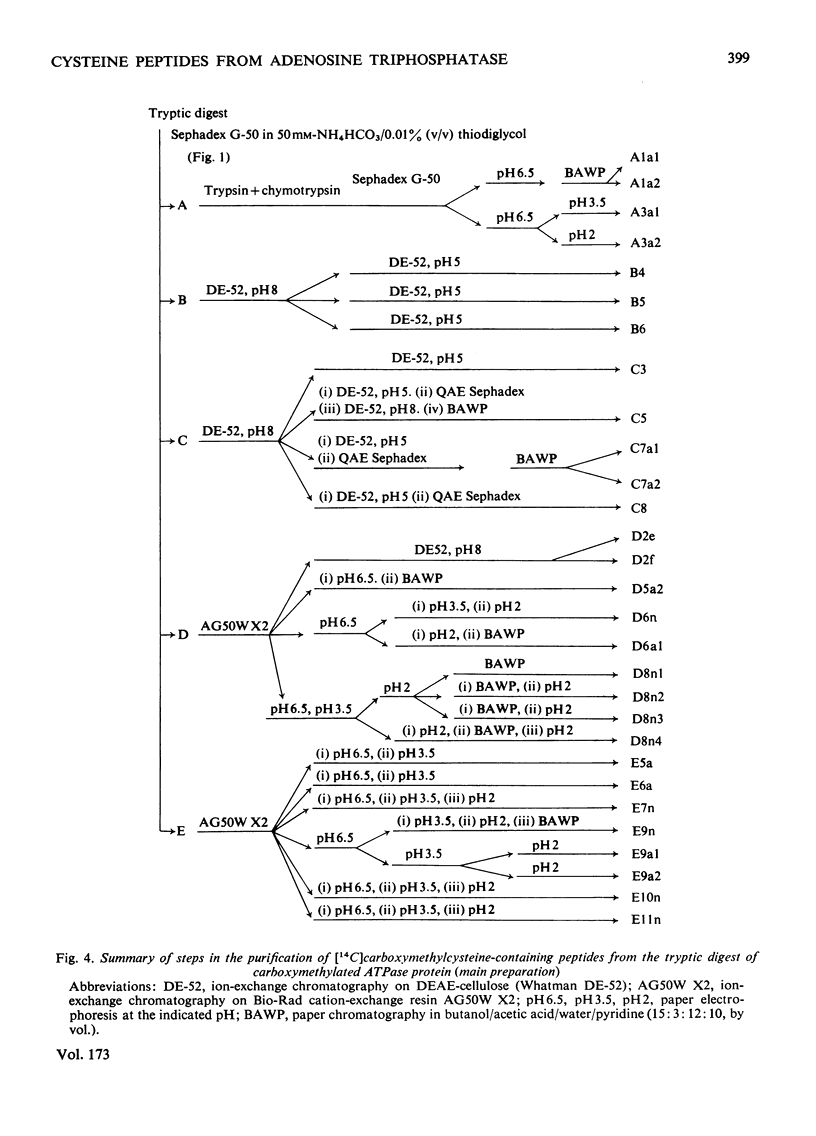
A preliminary investigation of the primary structure of the Ca(2+-transporting ATPase (adenosine triphosphatase) protein of rabbit skeletal-muscle sarcoplasmic reticulum is reported. The preparation of derivatives of delipidated protein in a form suitable for sequence analysis is described. Tryptic peptides containing S-carboxymethylcysteine residues were isolated from the reduced carboxymethylated protein, and their sequences were partially determined. The results are consistent with mol.wt. about 105000 for the polypeptide, and the absence of extended repeated lengths of sequence. The distribution of tryptophan and cysteine residues between large, aggregated peptides and soluble tryptic peptides shows that these residues are concentrated in different regions of the primary structure. This observation agrees with other evidence that these residues are, on the whole, widely separated in the native protein. The details of the procedures used to isolate the peptides, and the evidence for the determination of their sequences, are given Supplementary Publication SUP 50085 (30 pages), which has been deposited at the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem.J. (1978) 169, 5.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Coan C. R., Inesi G. Ca2+-dependent effect of ATP on spin-labeled sarcoplasmic reticulum. J Biol Chem. 1977 May 10;252(9):3044–3049. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hardwicke M. D. The binding of lipid to the lipid-free adenosine triphosphatase protein of sarcoplasmic reticulum. Eur J Biochem. 1976 Mar 1;62(3):431–438. doi: 10.1111/j.1432-1033.1976.tb10176.x. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C., Shooter E. M. The proteins of rabbit skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Dec;153(2):641–655. doi: 10.1016/0003-9861(72)90383-9. [DOI] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Arch Biochem Biophys. 1971 Aug;145(2):456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. J., Maire M., Reynolds J. A., Tanford C. Molecular weights and hydrophobicity of the polypeptide chain of sarcoplasmic reticulum calcium(II) adenosine triphosphatase and of its primary tryptic fragments. Biochemistry. 1976 Aug 10;15(16):3433–3437. doi: 10.1021/bi00661a006. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Separation and characterisation of tryptic fragments from the adenosine triphosphatase of sarcoplasmic reticulum. Eur J Biochem. 1975 Nov 1;59(1):193–200. doi: 10.1111/j.1432-1033.1975.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]


