Abstract
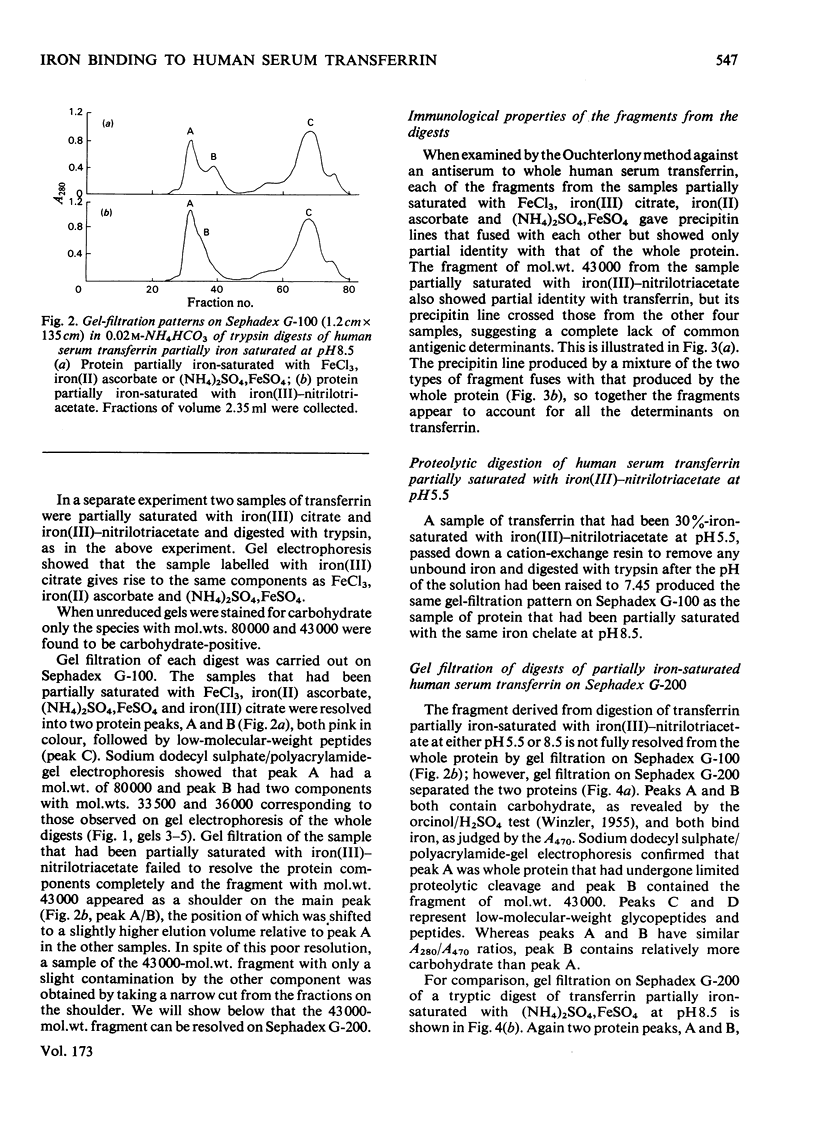
1. Trypsin digestion of human serum transferrin partially saturated with iron(III)-nitrilotriacetate at pH 5.5 or pH 8.5 produces a carbohydrate-containing iron-binding fragment of mol.wt. 43000. 2. When iron(III) citrate, FeCl3, iron (III) ascorabate and (NH4)2SO4,FeSO4 are used as iron donors to saturate the protein partially, at pH8.5, proteolytic digestion yields a fragment of mol.wt. 36000 that lacks carbohydrate. 3. The two fragments differ in their antigenic structures, amino acid compositions and peptide 'maps'. 4. The fragment with mol.wt. 36000 was assigned to the N-terminal region of the protein and the other to the C-terminal region. 5. The distribution of iron in human serum transferrin partially saturated with various iron donors was examined by electrophoresis in urea/polyacrylamide gels and the two possible monoferric forms were unequivocally identified. 6. The site designated A on human serum transferrin [Harris (1977) Biochemistry 16, 560--564] was assigned to the C-terminal region of the protein and the B site to the N-terminal region. 7. The distribution of iron on transferrin in human plasma was determined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A., Reich H. A. Studies on the binding of iron to transferrin and conalbumin. J Biol Chem. 1966 Apr 25;241(8):1666–1671. [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Arzabe F. R. Cleavage of differic bovine transferrin into two monoferric fragments. FEBS Lett. 1976 Oct 15;69(1):63–66. doi: 10.1016/0014-5793(76)80654-0. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Arzabe F., Lampreave F., Piñeiro A. The effect of trypsin on bovine transferrin and lactoferrin. Biochim Biophys Acta. 1976 Sep 28;446(1):214–225. doi: 10.1016/0005-2795(76)90112-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B., SALTMAN P., BENSON S. The stability constants of the iron-transferrin complex. Biochem Biophys Res Commun. 1962 Jun 19;8:56–60. doi: 10.1016/0006-291x(62)90235-8. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Significance of the binding of iron by transferrin. Nature. 1967 Aug 5;215(5101):584–586. doi: 10.1038/215584a0. [DOI] [PubMed] [Google Scholar]
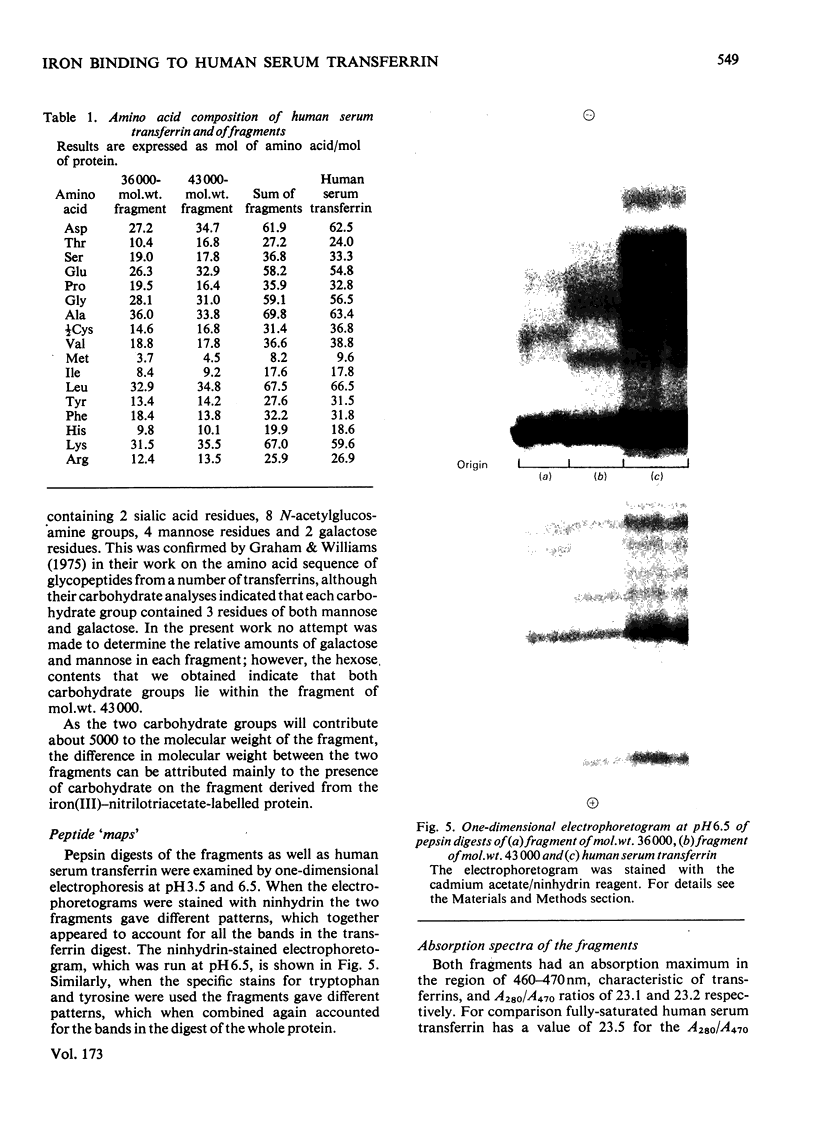
- Graham I., Williams J. A comparsion of glycopeptides from the transferrins of several species. Biochem J. 1975 Feb;145(2):263–279. doi: 10.1042/bj1450263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Harris D. C. Different metal-binding properties of the two sites of human transferrin. Biochemistry. 1977 Feb 8;16(3):560–564. doi: 10.1021/bi00622a033. [DOI] [PubMed] [Google Scholar]
- Harris D. C. Functional equivalence of iron bound to human transferrin at low pH or high pH. Biochim Biophys Acta. 1977 Feb 28;496(2):563–565. doi: 10.1016/0304-4165(77)90338-5. [DOI] [PubMed] [Google Scholar]
- JAMIESON G. A. STUDIES ON GLYCOPROTEINS. II. ISOLATION OF THE CARBOHYDRATE CHAINS OF HUMAN TRANSFERRIN. J Biol Chem. 1965 Jul;240:2914–2920. [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Lane R. S. Differences between human Fe1-transferrin molecules. Br J Haematol. 1975 Mar;29(3):511–520. doi: 10.1111/j.1365-2141.1975.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Matson G. A., Sutton H. E., Swanson J., Robinson A. R., Santiana A. Distribution of hereditary blood groups among Indians in South America. I. In Ecuador. Am J Phys Anthropol. 1966 Jan;24(1):51–69. doi: 10.1002/ajpa.1330240107. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Difference between the two iron-binding sites of transferrin. Nature. 1975 May 1;255(5503):87–88. doi: 10.1038/255087a0. [DOI] [PubMed] [Google Scholar]
- Roop W. E., Putnam F. W. Purification and properties of human transferrin C and a slow moving genetic variant. J Biol Chem. 1967 May 25;242(10):2507–2513. [PubMed] [Google Scholar]
- WARNER R. C., WEBER I. The preparation of crystalline conalbumin. J Biol Chem. 1951 Jul;191(1):173–180. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- Williams J. Iron-binding fragments from the carboxyl-terminal region of hen ovotransferrin. Biochem J. 1975 Jul;149(1):237–244. doi: 10.1042/bj1490237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. The formation of iron-binding fragments of hen ovotransferrin by limited proteolysis. Biochem J. 1974 Sep;141(3):745–752. doi: 10.1042/bj1410745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]