Abstract
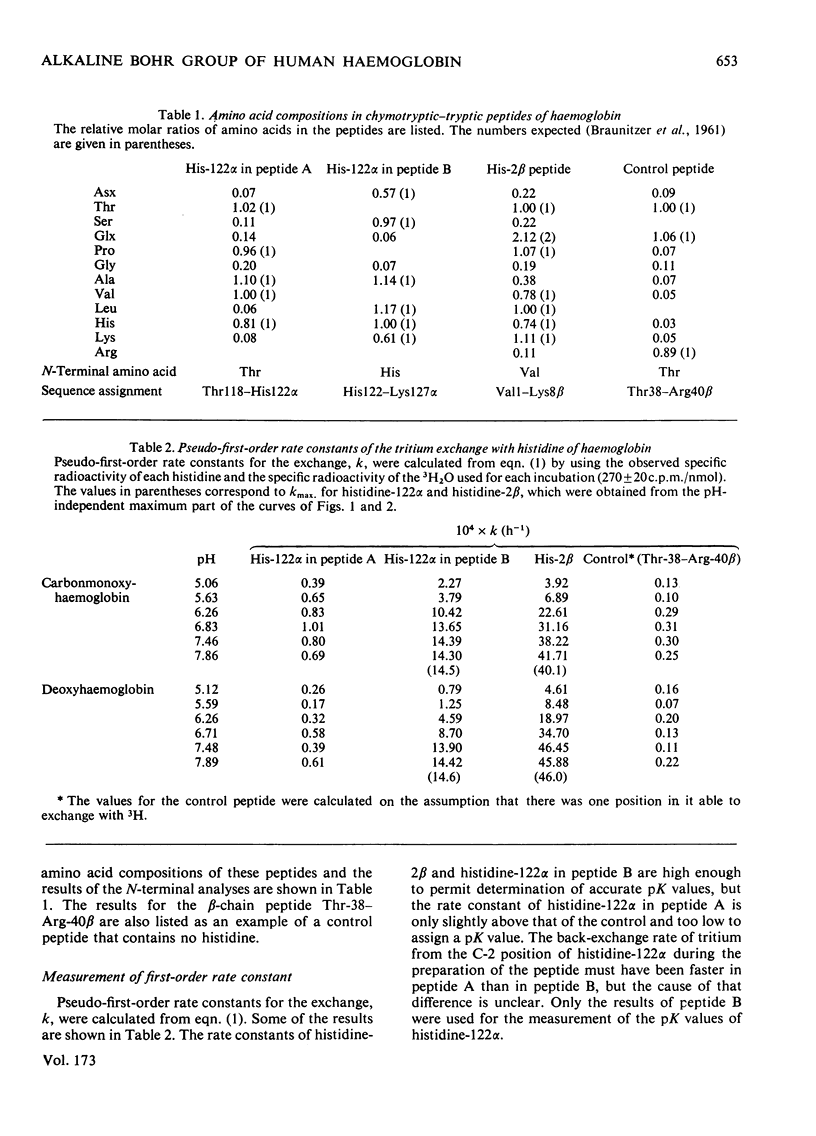
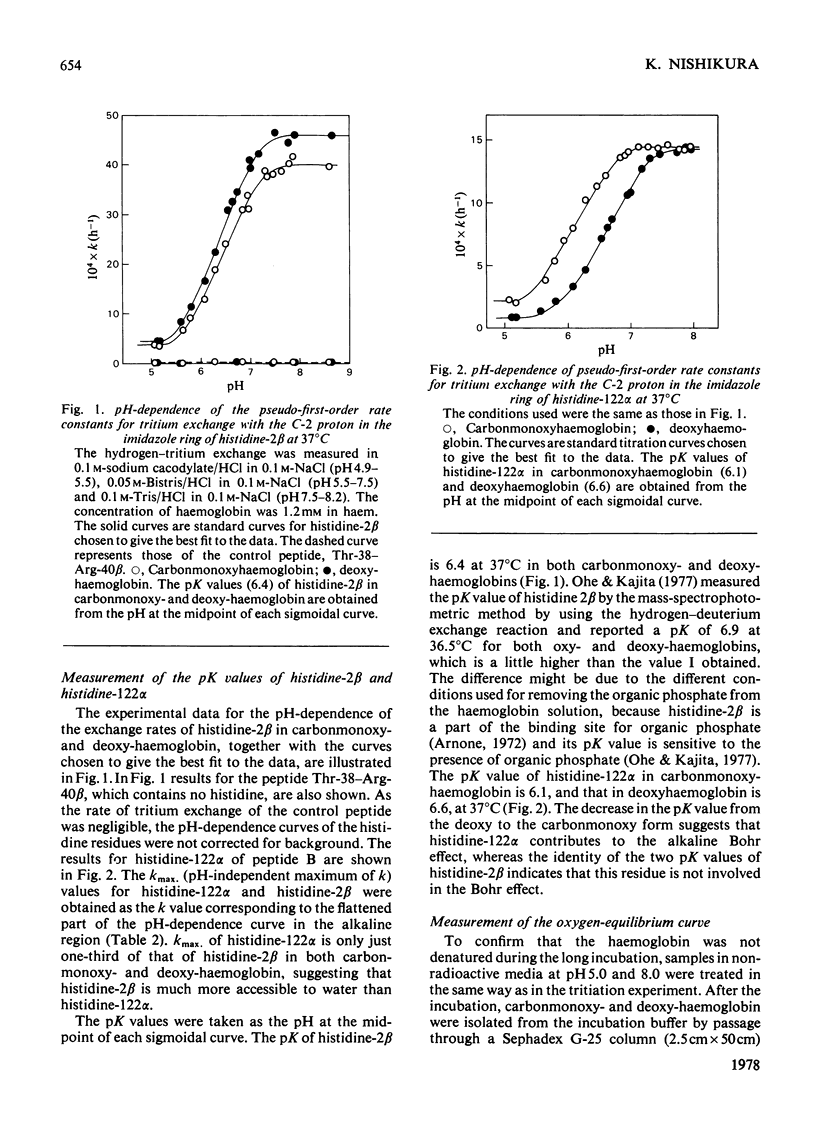
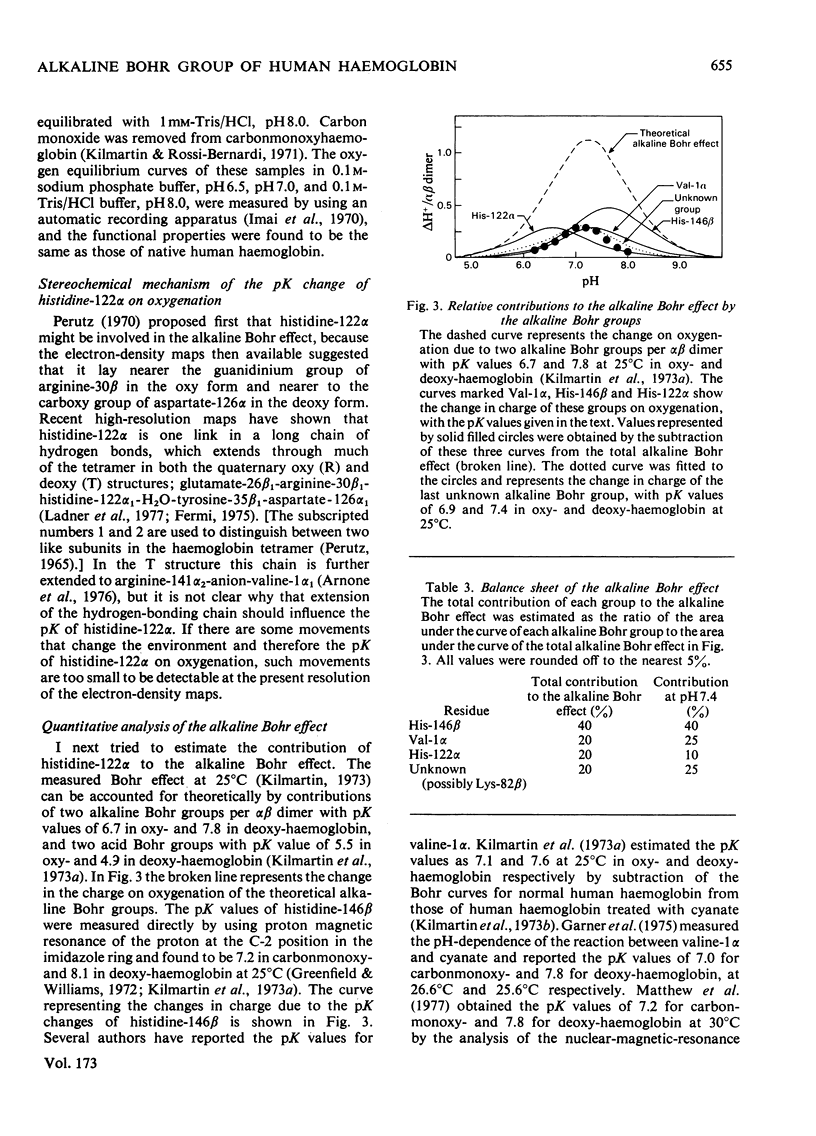
Human carbonmonoxy- and deoxy-haemoglobins were incubated at 37 degrees C in 3H2O at various pH values to measure the pH-dependent hydrogen--tritium exchange at the C-2 position of the imidazole ring of histidine-122alpha. To obtain the pseudo-first-order rate constants for the exchange, k, the two peptides containing histidine-122alpha were isolated and the amounts of tritium incorporated were determined. The rate constants gave pK values for the histidine of 6.1 in carbonmonoxyhaemoglobin and 6.6 in deoxyhaemoglobin, showing that it contributes about 20% to the total alkaline Bohr effect and about 10% at pH7.4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., GEHRING-MUELLER R., HILSCHMANN N., HILSE K., HOBOM G., RUDLOFF V., WITTMANN-LIEBOLD B. [The structure of normal adult human hemoglobins]. Hoppe Seylers Z Physiol Chem. 1961 Sep 20;325:283–286. doi: 10.1515/bchm2.1961.325.1.283. [DOI] [PubMed] [Google Scholar]
- Brunori M. Molecular adaptation to physiological requirements: the hemoglobin system of trout. Curr Top Cell Regul. 1975;9:1–39. doi: 10.1016/b978-0-12-152809-6.50008-1. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Bogardt R. A., Jr, Gurd F. R. Determination of the pK values for the alpha-amino groups of human hemoglobin. J Biol Chem. 1975 Jun 25;250(12):4398–4404. [PubMed] [Google Scholar]
- Greenfield N. J., Williams M. N. Proton magnetic resonance studies of human hemoglobin. Histidine titrations. Biochim Biophys Acta. 1972 Feb 29;257(2):187–197. doi: 10.1016/0005-2795(72)90270-x. [DOI] [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Breen J. J., Roberts G. C., Ho C. Direct measurement of the pK values of an alkaline Bohr group in human hemoglobin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1246–1249. doi: 10.1073/pnas.70.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Fogg J., Luzzana M., Rossi-Bernardi L. Role of the alpha-amino groups of the alpha and beta chains of human hemoglobin in oxygen-linked binding of carbon dioxide. J Biol Chem. 1973 Oct 25;248(20):7039–7043. [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol Rev. 1973 Oct;53(4):836–890. doi: 10.1152/physrev.1973.53.4.836. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V. The interaction of inositol hexaphosphate with methaemoglobin. Biochem J. 1973 Aug;133(4):725–733. doi: 10.1042/bj1330725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Koeppe R. E., 2nd, Stroud R. M. pH dependence of tritium exchange with the C-2 protons of the histidines in bovine trypsin. Biochemistry. 1976 Aug 10;15(16):3458–3464. doi: 10.1021/bi00661a010. [DOI] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Morrow J. S., Wittebort R. J., Gurd F. R. Quantitative determination of carbamino adducts of alpha and beta chains in human adult hemoglobin in presence and absence of carbon monoxide and 2,3-diphosphoglycerate. J Biol Chem. 1977 Apr 10;252(7):2234–2244. [PubMed] [Google Scholar]
- Oe M., Matsuo H., Sakiyama F., Narita K. Determination of pKa's of individual histidine residues in pancreatic ribonuclease by hydrogen-tritium exchange. J Biochem. 1974 May;75(5):1197–1200. doi: 10.1093/oxfordjournals.jbchem.a130496. [DOI] [PubMed] [Google Scholar]
- Ohe M., Kajita A. Studies on the heterotropic interaction of hemoglobin. II. Role of beta-146 and beta-2 histidines in the alkaline Bohr effect. J Biochem. 1977 Sep;82(3):839–845. doi: 10.1093/oxfordjournals.jbchem.a131759. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Rossi-Bernardi L., Roughton F. J. The effect of temperature on the oxygen-linked ionizations of hemoglobin. J Biol Chem. 1967 Mar 10;242(5):784–792. [PubMed] [Google Scholar]
- Tuchinda S., Nagai K., Lehmann H. Oxygen dissociation curve of haemoglobin Portland. FEBS Lett. 1975 Jan 1;49(3):390–391. doi: 10.1016/0014-5793(75)80792-7. [DOI] [PubMed] [Google Scholar]


