Abstract
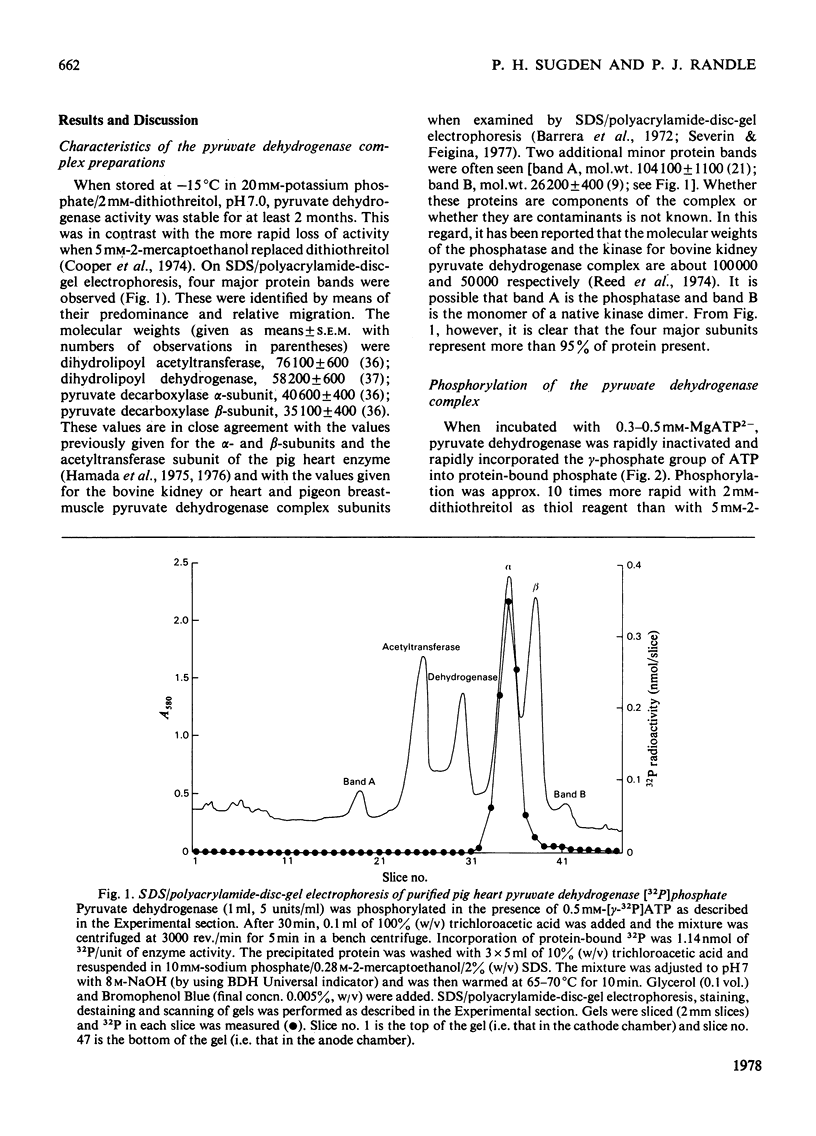
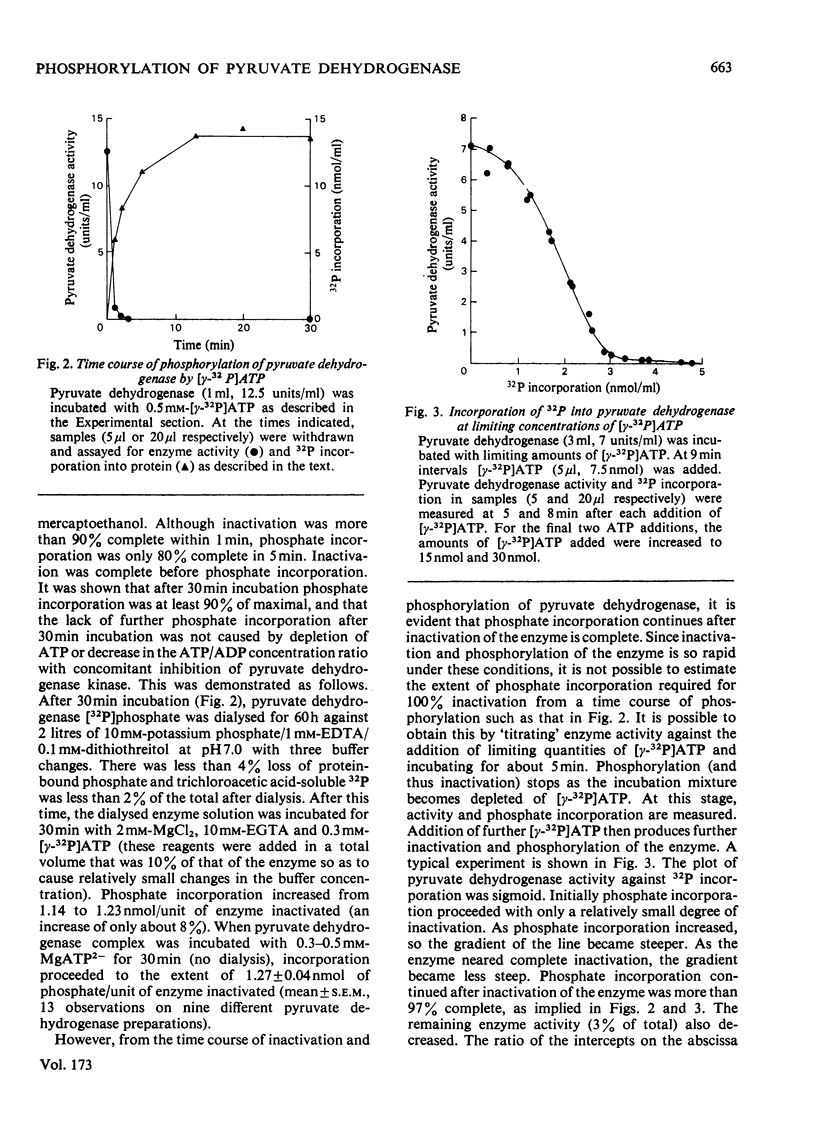
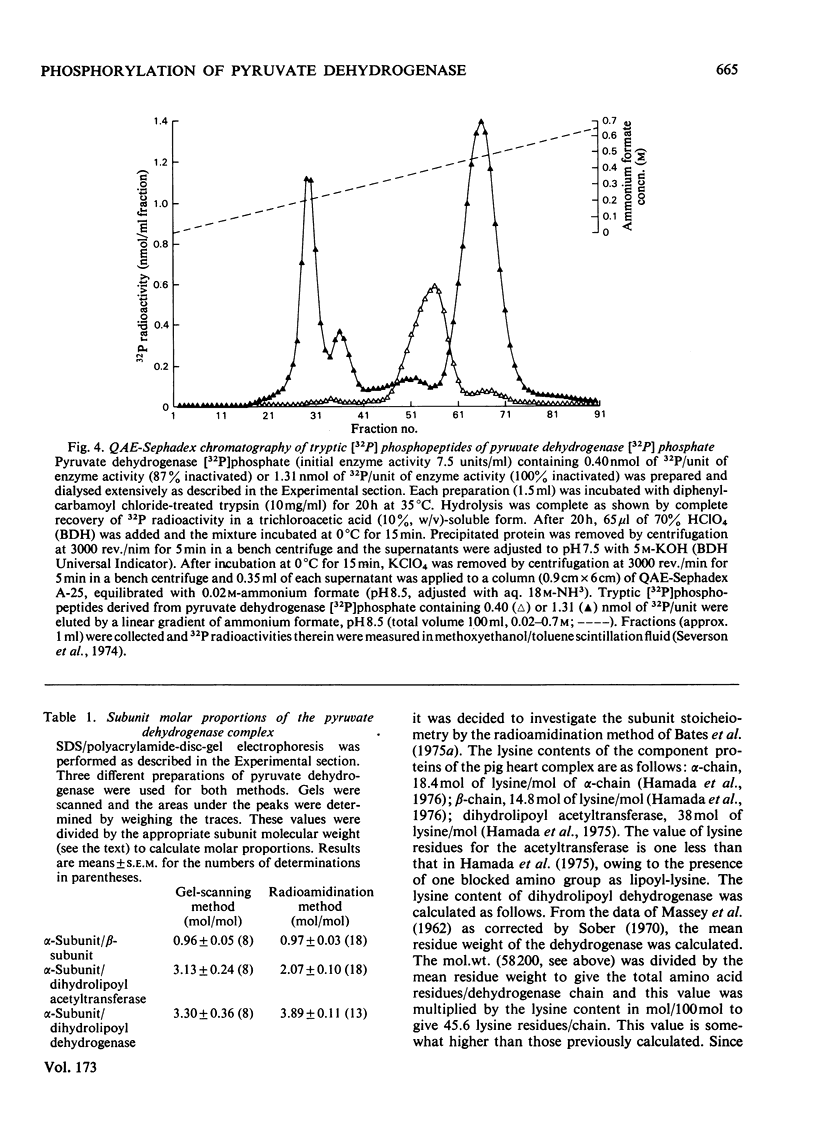
1. The molecular weights of the subunits of purified pig heart pyruvate dehydrogenase complex were determined by sodium dodecyl sulphate/polyacrylamide-disc-gel electrophoresis and were: pyruvate decarboxylase, α-subunit 40600, β-subunit 35100; dihydrolipoyl acetyltransferase 76100; dihydrolipoyl dehydrogenase 58200. 2. Inactivation of the pyruvate dehydrogenase complex by its integral kinase corresponded to the incorporation of 0.46nmol of P/unit of complex activity inactivated. 3. Further incorporation of phosphate into the complex occurred to a limit of 1.27nmol of P/unit of complex inactivated (approx. 3 times that required for inactivation). 4. Phosphate was incorporated only into the α-subunit of the decarboxylase. 5. The molar ratio of phosphate to α-subunits of the decarboxylase was estimated by radioamidination of amino groups of pyruvate dehydrogenase [32P]phosphate complex by using methyl [1-14C]acetimidate, followed by separation of α-subunits by sodium dodecyl sulphate/polyacrylamide-disc-gel electrophoresis. Inactivation of the complex (0.46nmol of P/unit of complex inactivated) corresponded to a molar ratio of one phosphate group per two α-chains (i.e. one phosphate group/α2β2 tetramer). Complete phosphorylation corresponded to three phosphate groups per α2β2 tetramer. 6. Subunit molar ratios in the complex were also estimated by the radioamidination technique. Results corresponded most closely to molar ratios of 4 α-subunits:4 β-subunits:2 dihydrolipoyl acetyltransferase subunits:1 dihydrolipoyl dehydrogenase subunit.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Harrison R. A., Perham R. N. The stoichiometry of polypeptide chains in the pyruvate dehydrogenase multienzyme complex of E. coli determined by a simple novel method. FEBS Lett. 1975 Dec 15;60(2):427–430. doi: 10.1016/0014-5793(75)80764-2. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Butler J. R., Pettit R. H., Davis P. F., Reed L. J. Binding of thiamin thiazolone pyrophosphate to mammalian pyruvate dehydrogenase and its effects of kinase and phosphatase activities. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1667–1674. doi: 10.1016/0006-291x(77)90636-2. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Davis P. F., Pettit F. H., Reed L. J. Peptides derived from pyruvate dehydrogenase as substrates for pyruvate dehydrogenase kinase and phosphatase. Biochem Biophys Res Commun. 1977 Apr 11;75(3):541–549. doi: 10.1016/0006-291x(77)91506-6. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. N. Quantitative densitometry of 1-50 g protein in acrylamide gel slabs with Coomassie blue. Anal Biochem. 1972 Apr;46(2):388–401. doi: 10.1016/0003-2697(72)90312-0. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hamada M., Hiraoka T., Koike K., Ogasahara K., Kanzaki T. Properties and subunit structure of pig heart pyruvate dehydrogenase. J Biochem. 1976 Jun;79(6):1273–1285. doi: 10.1093/oxfordjournals.jbchem.a131181. [DOI] [PubMed] [Google Scholar]
- Hamada M., Otsuka K. I., Tanaka N., Ogasahara K., Koike K. Purification properties and subunit composition of pig heart lipoate acetyltransferase. J Biochem. 1975 Jul;78(1):187–197. [PubMed] [Google Scholar]
- Hayakawa T., Kanzaki T., Kitamura T., Fukuyoshi Y., Sakurai Y., Koike K., Suematsu T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. V. Resolution and reconstitution studies of the pig heart pyruvate dehydrogenase complex. J Biol Chem. 1969 Jul 10;244(13):3660–3670. [PubMed] [Google Scholar]
- Ishikawa E., Oliver R. M., Reed L. J. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):534–541. doi: 10.1073/pnas.56.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V., HOFMANN T., PALMER G. The relation of function and structure in lipoyl dehydrogenase. J Biol Chem. 1962 Dec;237:3820–3828. [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- Riley M., Perham R. N. The reversible reaction of protein amino groups with exo-cis-3,6-endoxo-delta-tetrahydrophthalic anhydride. Biochem J. 1970 Aug;118(5):733–739. doi: 10.1042/bj1180733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin S. E., Feigina M. M. alpha-keto acid dehydrogenases and acyl-CoA synthetases from pigeon breast muscle. Adv Enzyme Regul. 1976;15:1–21. doi: 10.1016/0065-2571(77)90006-1. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling T. R. Regulation of glycogen synthetase. Specificity and stoichiometry of phosphorylation of the skeletal muscle enzyme by cyclic 3':5'-AMP-dependent protein kinase. J Biol Chem. 1975 Jul 25;250(14):5407–5412. [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Cooper R. H., Denton R. M., Bridges B. J., Randle P. J. The elementary reactions of the pig heart pyruvate dehydrogenase complex. A study of the inhibition by phosphorylation. Biochem J. 1976 Jul 1;157(1):41–67. doi: 10.1042/bj1570041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wieland O., Funcke H. v., Löffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett. 1971 Jul 1;15(4):295–298. doi: 10.1016/0014-5793(71)80641-5. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]


