Abstract
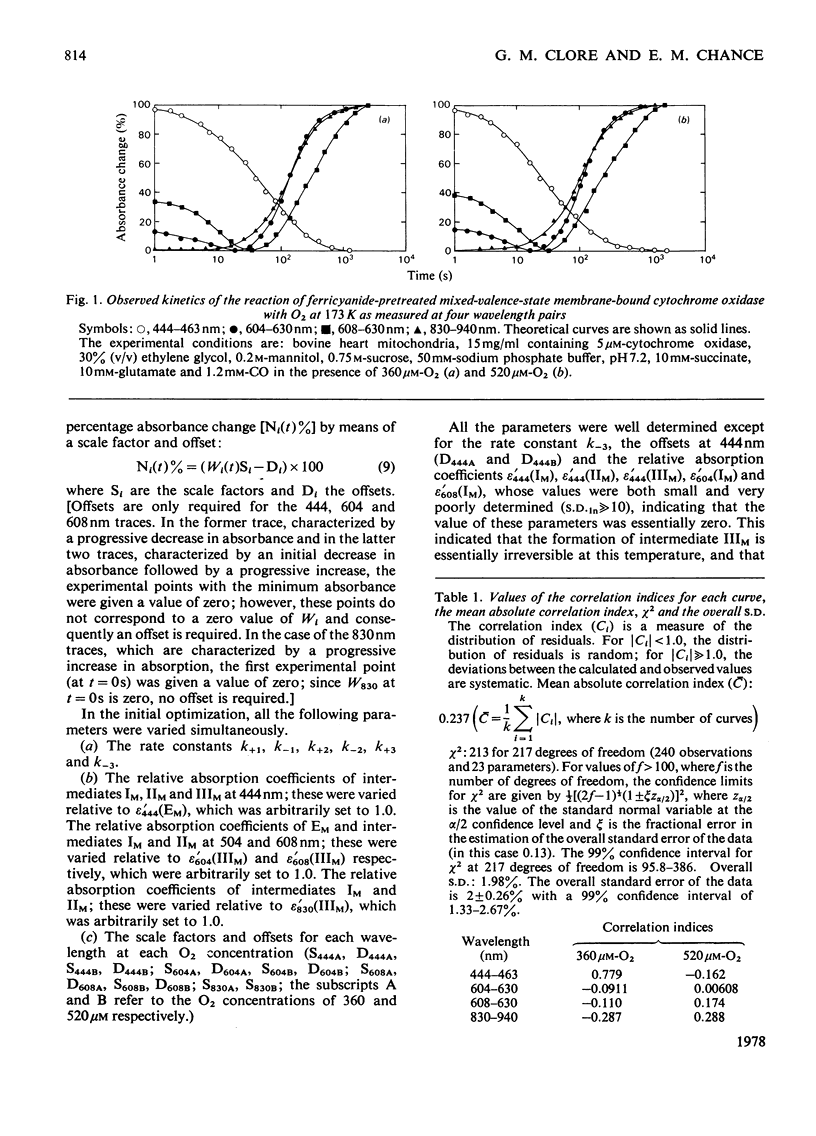
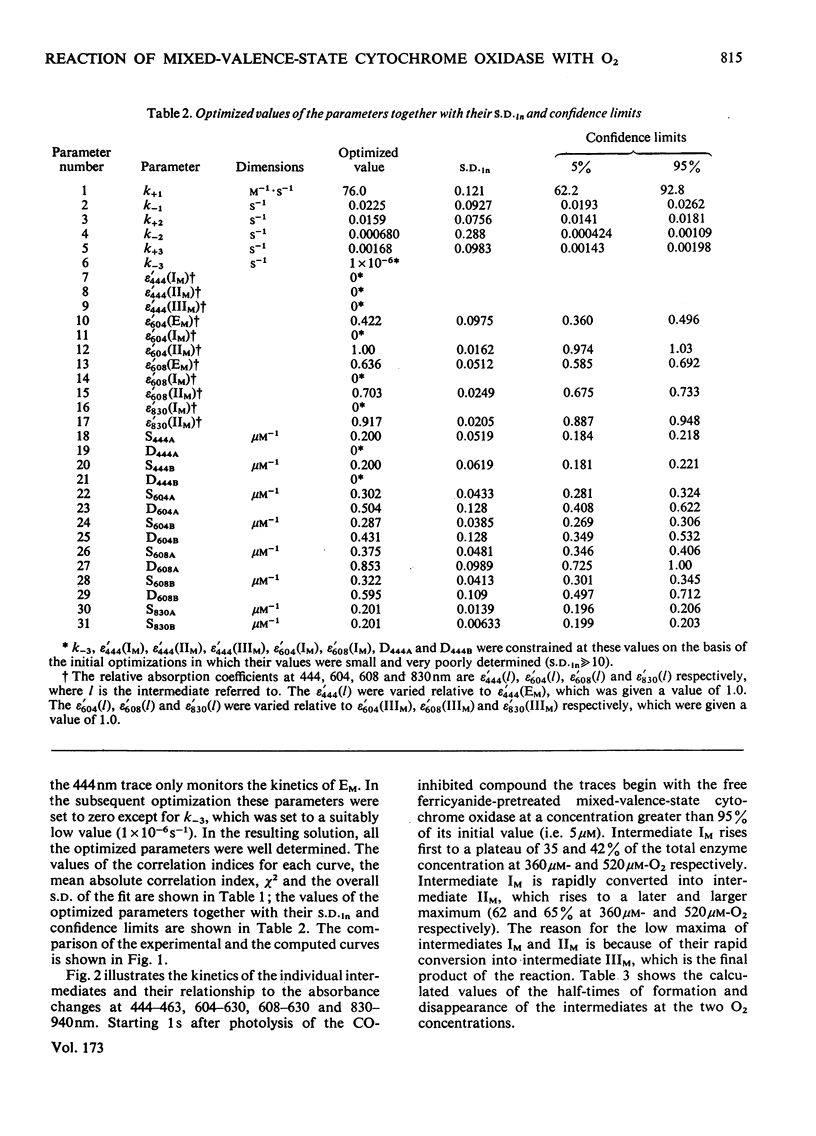
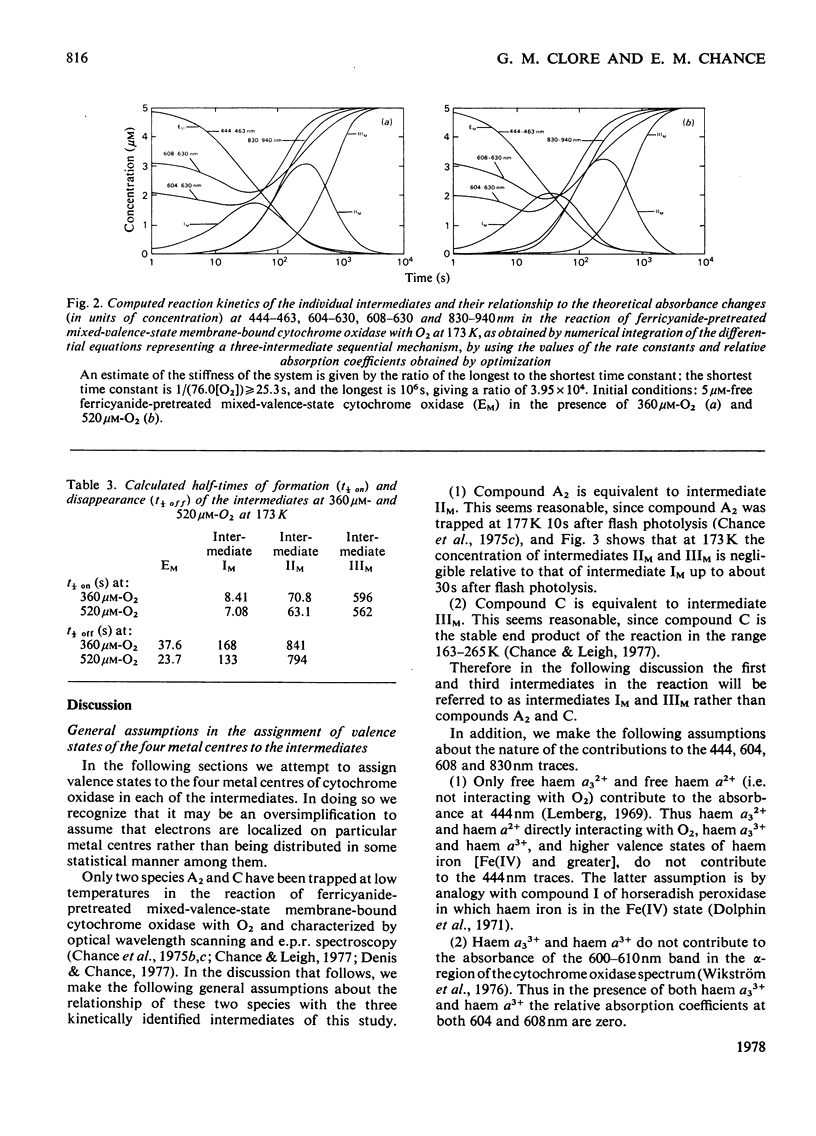
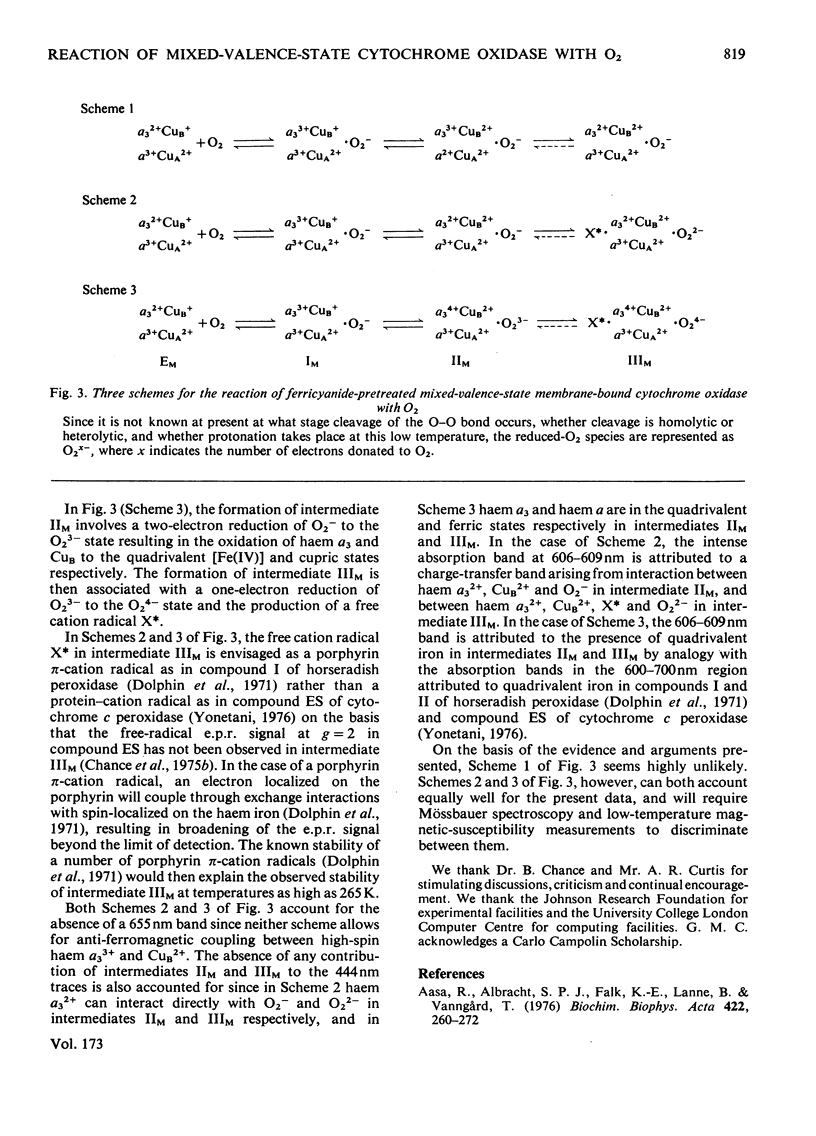
1. The results of non-linear optimization studies on the mechanism of reaction of ferricyanide-pretreated mixed-valence-state cytochrome oxidase with O2 at 173 K are presented. The analysis is carried out on data obtained by means of dual-wavelength multi-channel spectroscopy at four wavelength pairs (444-463 nm, 604-630 nm, 608-630 nm and 830-940 nm) and at two O2 concentrations (360 micron and 520 micron). The only model that satisfies the triple requirement of a standard deviation within the standard error of the experimental data, a random distribution of residuals and good determination of the optimized parameters, is a three-intermediate sequential mechanism. 2. On the basis of the optimized values of the relative absorption coefficients of the intermediates at each wavelength obtained from the present paper together with data from optical wavelength scanning and e.p.r. spectroscopy obtained by low-temperature trapping studies, the possible valence states of the metal centres in each of the intermediates are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Beinert H., Hansen R. E., Hartzell C. R. Kinetic studies on cytochrome c oxidase by combined epr and reflectance spectroscopy after rapid freezing. Biochim Biophys Acta. 1976 Feb 16;423(2):339–355. doi: 10.1016/0005-2728(76)90190-0. [DOI] [PubMed] [Google Scholar]
- Chance B., Legallais V., Sorge J., Graham N. A versatile time-sharing multichannel spectrophotometer, reflectometer, and fluorometer. Anal Biochem. 1975 Jun;66(2):498–514. doi: 10.1016/0003-2697(75)90617-x. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr Oxygen intermediates and mixed valence states of cytochrome oxidase: infrared absorption difference spectra of compounds A, B, and C of cytochrome oxidase and oxygen. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4777–4780. doi: 10.1073/pnas.74.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in reaction of cytochrome oxidase with oxygen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1635–1640. doi: 10.1073/pnas.72.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr, Ingledew W. J., King T. E. Low-temperature kinetics of the reaction of oxygen and solubilized cytochrome oxidase. Biochem J. 1978 Jun 1;171(3):787–798. doi: 10.1042/bj1710787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of fully reduced membrane-bound cytochrome oxidase with oxygen at 176K. Biochem J. 1978 Sep 1;173(3):799–810. doi: 10.1042/bj1730799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K. E., Vänngård T., Angström J. Heme spin-states of cytochrome c oxidase derived from room temperature magnetic susceptibility measurements. FEBS Lett. 1977 Mar 15;75(1):23–27. doi: 10.1016/0014-5793(77)80044-6. [DOI] [PubMed] [Google Scholar]
- Lanne B., Malmström B. G., Vänngård T. Effects of hexacyanoferrate on cytochrome c oxidase. FEBS Lett. 1977 May 15;77(2):146–150. doi: 10.1016/0014-5793(77)80222-6. [DOI] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- Lindsay J. G., Owen C. S., Wilson D. F. The invisible copper of cytochrome c oxidase. pH and ATP dependence of its midpoint potential and its role in the oxygen reaction. Arch Biochem Biophys. 1975 Aug;169(2):492–505. doi: 10.1016/0003-9861(75)90192-7. [DOI] [PubMed] [Google Scholar]
- Lindsay J. G., Wilson D. F. Reaction of cytochrome C oxidase with CO: involvement of the invisible copper. FEBS Lett. 1974 Nov 1;48(1):45–49. doi: 10.1016/0014-5793(74)81058-6. [DOI] [PubMed] [Google Scholar]
- Mackey L. N., Kuwana T., Hartzell C. R. Evaluation of the energetics of cytochrome C oxidase in the absense of cytochrome C. FEBS Lett. 1973 Nov 1;36(3):326–329. doi: 10.1016/0014-5793(73)80402-8. [DOI] [PubMed] [Google Scholar]
- Muijsers A. O., Tiesjema R. H., Henderson R. W., Van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VII. The effect of cytochrome c on the oxidation-reduction potential of isolated cytochrome aa 3 . Biochim Biophys Acta. 1972 Apr 20;267(1):216–221. doi: 10.1016/0005-2728(72)90154-5. [DOI] [PubMed] [Google Scholar]
- Palmer G., Babcock G. T., Vickery L. E. A model for cytochrome oxidase. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2206–2210. doi: 10.1073/pnas.73.7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. P. Variable-temperature magnetic-circular-dichroism spectra of cytochrome c oxidase and its derivatives. Biochem J. 1977 Aug 1;165(2):327–336. doi: 10.1042/bj1650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. Determination of the heme spin states in cytochrome c oxidase using magnetic circular dichroism. FEBS Lett. 1976 Aug 1;67(1):94–98. doi: 10.1016/0014-5793(76)80877-0. [DOI] [PubMed] [Google Scholar]
- VAN GELDERB, SLATER E. C. TITRATION OF CYTOCHROME C OXIDASE WITH NADH AND PHENAZINE METHOSULPHATE. Biochim Biophys Acta. 1963 Aug 6;73:663–665. doi: 10.1016/0006-3002(63)90342-1. [DOI] [PubMed] [Google Scholar]
- Wever R., Van Drooge J. H., Muijsers A. O., Bakker E. P., Van Gelker B. F. The binding of carbon monoxide to cytochrome c oxidase. Eur J Biochem. 1977 Feb 15;73(1):149–154. doi: 10.1111/j.1432-1033.1977.tb11301.x. [DOI] [PubMed] [Google Scholar]
- Wikström K. F., Harmon H. J., Ingledew W. J., Chance B. A re-evaluation of the spectral, potentiometric and energy-linked properties of cytochrome c oxidase in mitochondria. FEBS Lett. 1976 Jun 15;65(3):259–277. doi: 10.1016/0014-5793(76)80127-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Leigh J. S. Heme-heme interaction between the cytochromes of the mitochondrial respiratory chain. Ann N Y Acad Sci. 1974 Feb 18;227:630–635. doi: 10.1111/j.1749-6632.1974.tb14427.x. [DOI] [PubMed] [Google Scholar]


