Abstract
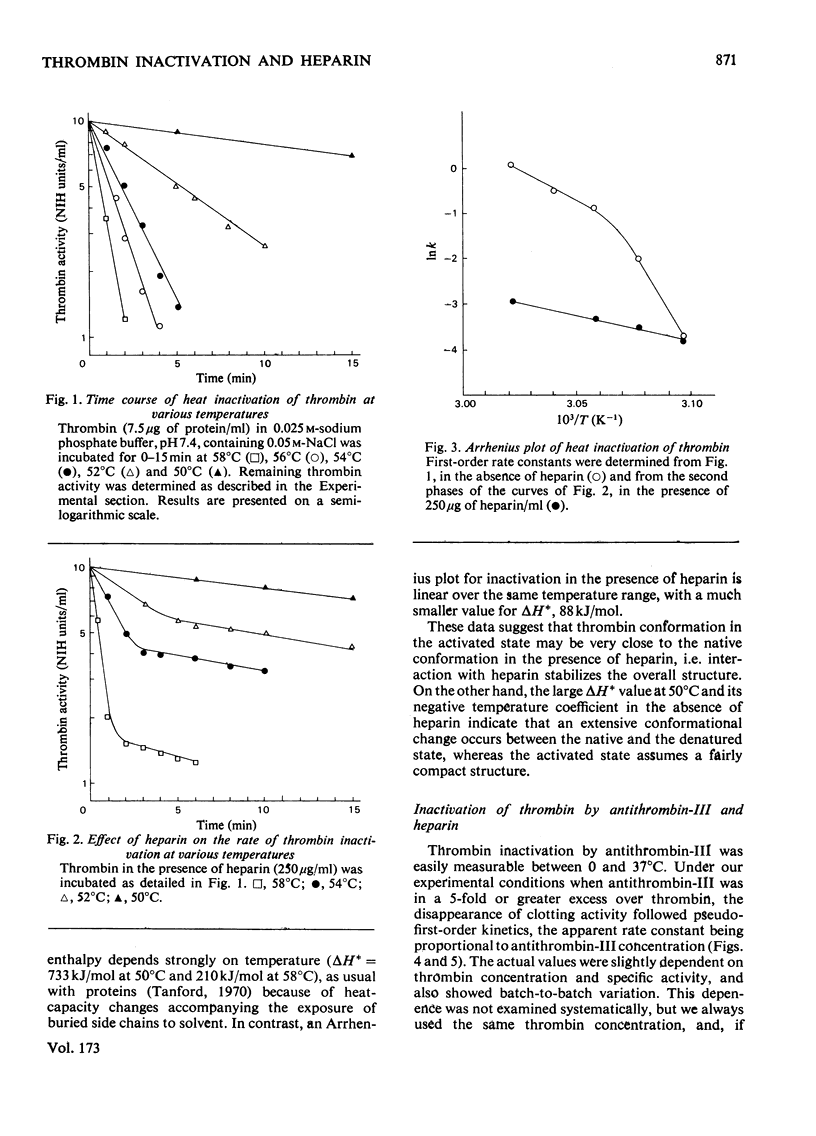
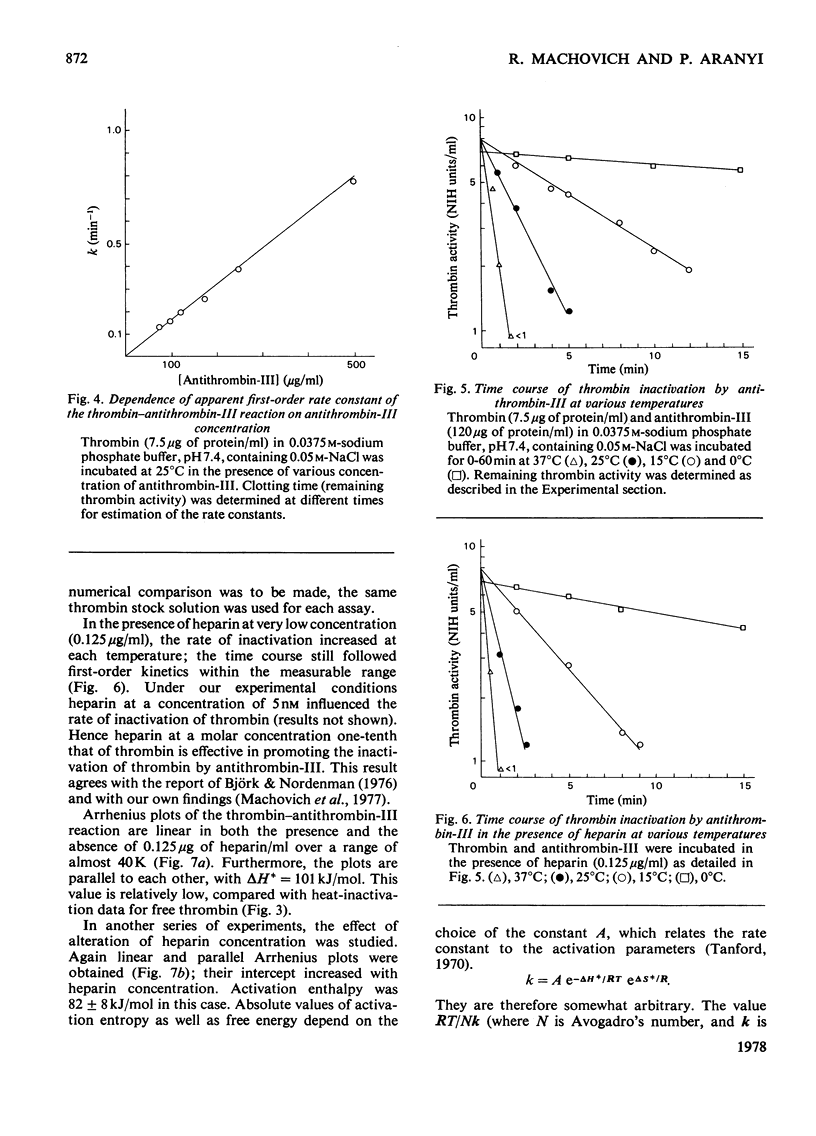
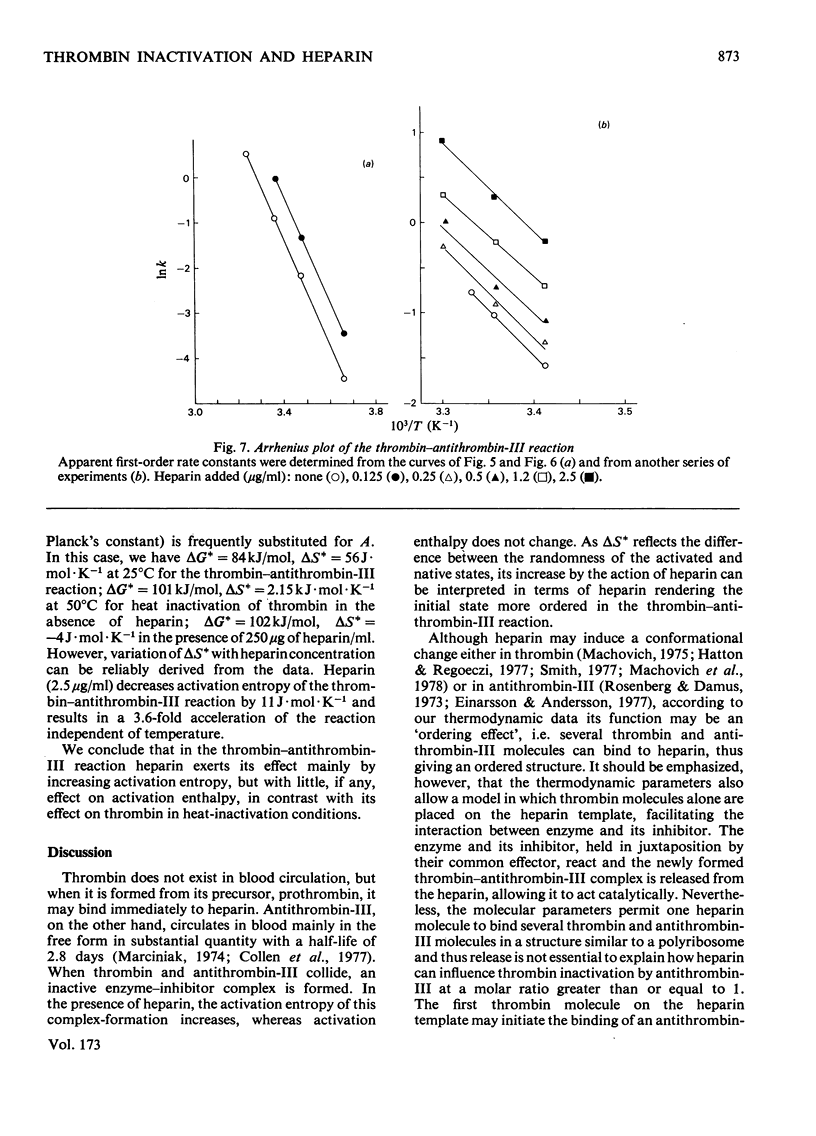
The inactivation of thrombin by heat and by its physiological inhibitor, antithrombin-III, shows quite different dependence on heparin concentration. Heparin at 250 microgram/ml protects thrombin against heat inactivation, and thrombin behaves heterogeneously in this reaction. In the absence of heparin, the thermodynamic activation parameters change with temperature (deltaH+ = 733 kJ/mol and 210 kJ/mol at 50 and 58 degrees C respectively). When heparin is present, heat inactivation of the protected thrombin species proceeds with deltaH+ = 88 kJ/mol and is independent of temperature in the same range. On the other hand, heparin at 0.125-2.5 microgram/ml accelerates the thrombin-antithrombin-III reaction. Thrombin does not show heterogeneity in this reaction and the time courses at any heparin concentration and any temperature between 0 and 37 degrees C appear to follow first-order kinetics. Activation enthalpy is independent of heparin concentration or temperature, deltaH+ = 82-101 kJ/mol, varying slightly with antithrombin-III concentration and thrombin specific activity. Heparin seems to exert its effect by increasing activation entropy. On the basis of these data we suggest a mechanism of action of heparin in the thrombin-antithrombin-III reaction which accounts for all the important features of the latter and seems to unify the different hypotheses that have been advanced.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U. Binding of thrombin to antithrombin III. Scand J Clin Lab Invest. 1969 Aug;24(1):23–27. doi: 10.3109/00365516909080127. [DOI] [PubMed] [Google Scholar]
- Abildgaard U. Highly purified antithrombin 3 with heparin cofactor activity prepared by disc electrophoresis. Scand J Clin Lab Invest. 1968;21(1):89–91. doi: 10.3109/00365516809076981. [DOI] [PubMed] [Google Scholar]
- Arányi P., Batke J., Machovich R. Remarks on the interaction of thrombim and heparin. Arch Biochem Biophys. 1977 Jun;181(2):678–679. doi: 10.1016/0003-9861(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Björk I., Nordenman B. Acceleration of the reaction between thrombin and antithrombin III by non-stoichiometric amounts of heparin. Eur J Biochem. 1976 Sep 15;68(2):507–511. doi: 10.1111/j.1432-1033.1976.tb10838.x. [DOI] [PubMed] [Google Scholar]
- Collen D., Schetz J., de Cock F., Holmer E., Verstraete M. Metabolism of antithrombin III (heparin cofactor) in man: effects of venous thrombosis and of heparin administration. Eur J Clin Invest. 1977 Feb;7(1):27–35. doi: 10.1111/j.1365-2362.1977.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- Danishefsky I., Ahrens M., Klein S. Effect of heparin modification on its activity in enhancing the inhibition of thrombin by antithrombin III. Biochim Biophys Acta. 1977 Jun 23;498(1):215–222. doi: 10.1016/0304-4165(77)90101-5. [DOI] [PubMed] [Google Scholar]
- Einarsson R., Andersson L. O. Binding of heparin to human antithrombin III as studied by measurements of tryptophan fluorescence. Biochim Biophys Acta. 1977 Jan 25;490(1):104–111. doi: 10.1016/0005-2795(77)90110-6. [DOI] [PubMed] [Google Scholar]
- Furugren B., Andersson L. O., Einarsson R. Small-angle x-ray scattering studies on human antithrombin III and its complex with heparin. Arch Biochem Biophys. 1977 Jan 30;178(2):419–424. doi: 10.1016/0003-9861(77)90211-9. [DOI] [PubMed] [Google Scholar]
- Gitel S. N. Evidence of a catalytic role of heparin in anticoagulation reactions. Adv Exp Med Biol. 1975;52:243–247. doi: 10.1007/978-1-4684-0946-8_20. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Regoeczi E. The inactivation of thrombin and plasmin by antithrombin III in the presence of sepharose-heparin. Thromb Res. 1977 May;10(5):645–660. doi: 10.1016/0049-3848(77)90047-0. [DOI] [PubMed] [Google Scholar]
- Hilborn J. C., Anastassiadis P. A. Acrylamide gel electrophoresis of acidic mucopolysaccharides. Anal Biochem. 1969 Oct 1;31(1):51–58. doi: 10.1016/0003-2697(69)90240-1. [DOI] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Li E. H., Orton C., Feinman R. D. The interaction of thrombin and heparin. Proflavine dye binding studies. Biochemistry. 1974 Nov 19;13(24):5012–5017. doi: 10.1021/bi00721a023. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Uhteg L. C., Vogel C. N., Kingdon H. S., Mann K. G. Preparation and partial characterization of two forms of bovine thrombin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):482–489. doi: 10.1016/0006-291x(75)90536-7. [DOI] [PubMed] [Google Scholar]
- Machovich R., Arányi P. Effect of calcium ion on the interaction between thrombin and heparin. Thermal denaturation. Thromb Haemost. 1977 Oct 31;38(3):677–684. [PubMed] [Google Scholar]
- Machovich R., Blaskó G., Pálos L. A. Action of heparin on thrombin-antithrombin reaction. Biochim Biophys Acta. 1975 Jan 30;379(1):193–200. doi: 10.1016/0005-2795(75)90022-7. [DOI] [PubMed] [Google Scholar]
- Machovich R., Borsodi A., Blaskó G., Orakzai S. A. Inactivation of alpha- and beta-thrombin by antithrombin-III, alpha 2-macroglobulin and alpha 1-proteinase inhibitor. Biochem J. 1977 Nov 1;167(2):393–398. doi: 10.1042/bj1670393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovich R. Mechanism of action of heparin through thrombin on blood coagulation. Biochim Biophys Acta. 1975 Nov 18;412(1):13–17. doi: 10.1016/0005-2795(75)90334-7. [DOI] [PubMed] [Google Scholar]
- Machovich R., Staub M., Patthy L. Decreased heparin sensitivity of cycholhexanedione-modified thrombin. Eur J Biochem. 1978 Feb;83(2):473–477. doi: 10.1111/j.1432-1033.1978.tb12113.x. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Yip R., Heldebrant C. M., Fass D. N. Multiple active forms of thrombin. 3. Polypeptide chain location of active site serine and carbohydrate. J Biol Chem. 1973 Mar 10;248(5):1868–1875. [PubMed] [Google Scholar]
- Marciniak E. Binding of heparin in vitro and in vivo to plasma proteins. J Lab Clin Med. 1974 Sep;84(3):344–356. [PubMed] [Google Scholar]
- McDuffie N. M., Dietrich C. P., Nader H. B. Electrofocusing of heparin: fractionation of heparin into 21 components distinguishable from other acidic mucopolysaccharides. Biopolymers. 1975 Jul;14(7):1473–1486. doi: 10.1002/bip.1975.360140713. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Yuan L., Stivala S. S. Three-dimensional model of heparin. Adv Exp Med Biol. 1975;52:39–49. doi: 10.1007/978-1-4684-0946-8_3. [DOI] [PubMed] [Google Scholar]


