Abstract
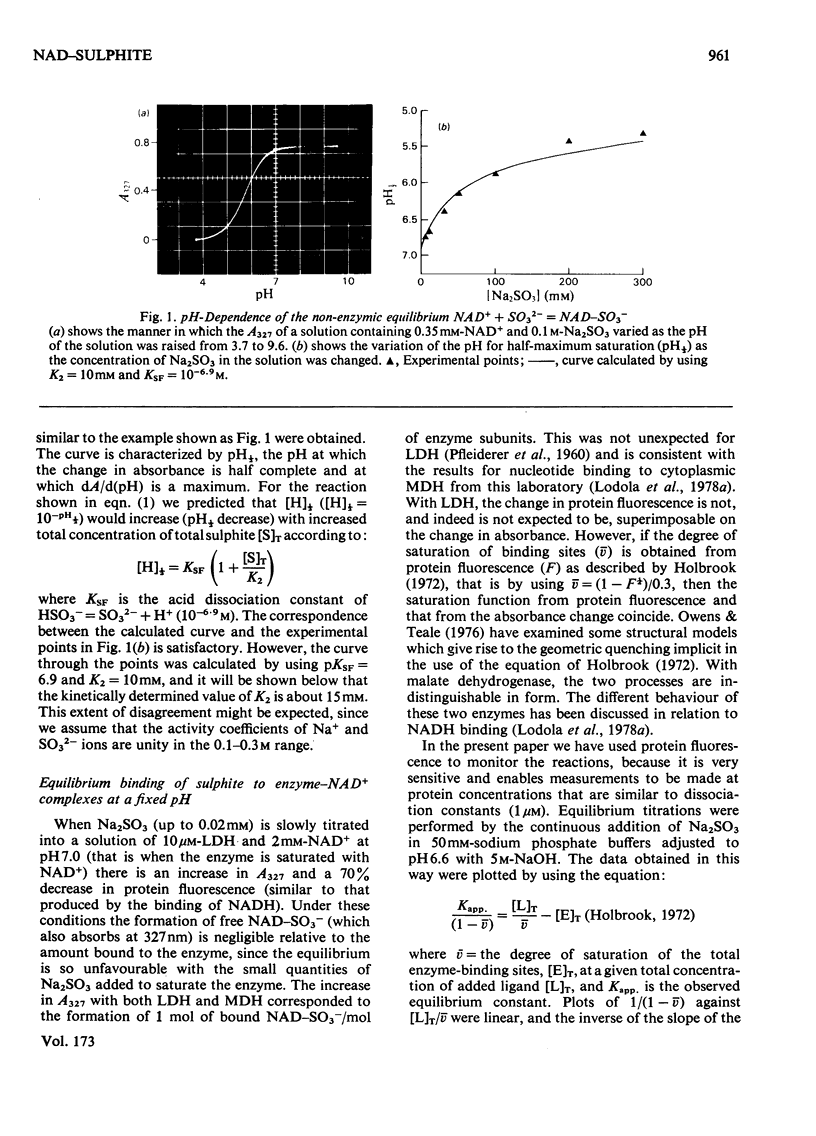
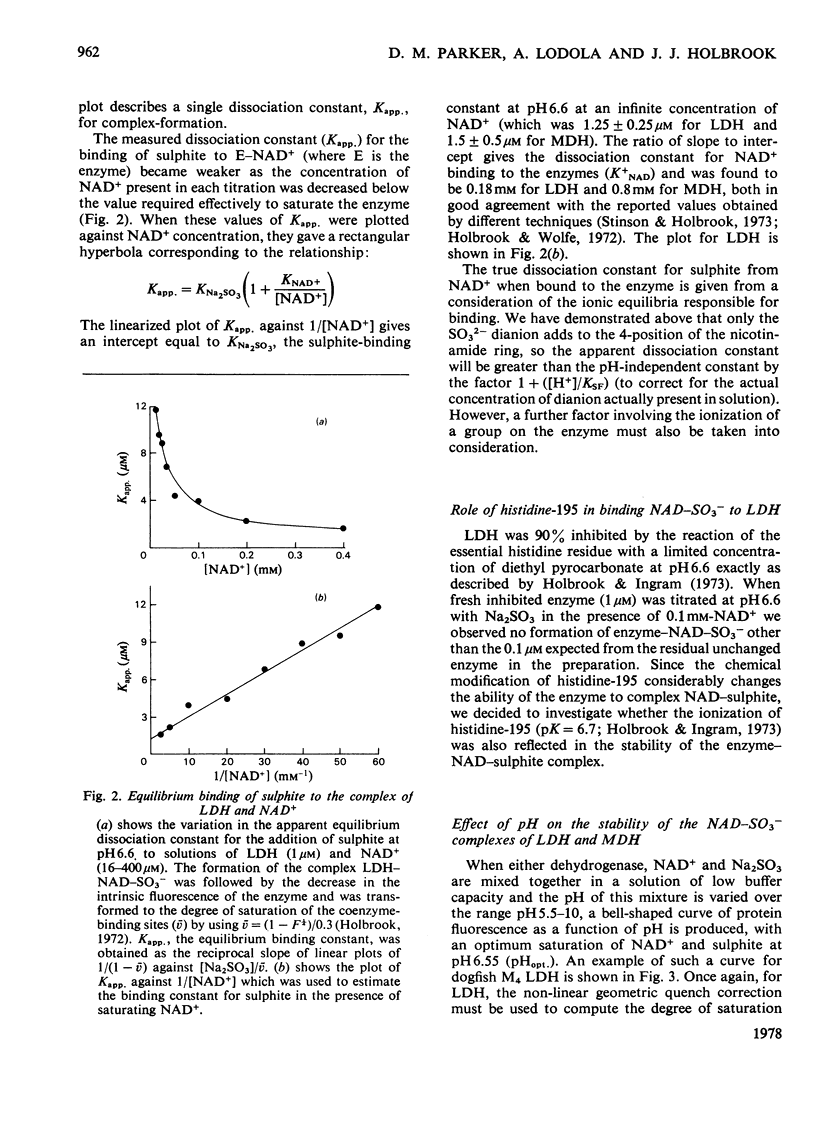
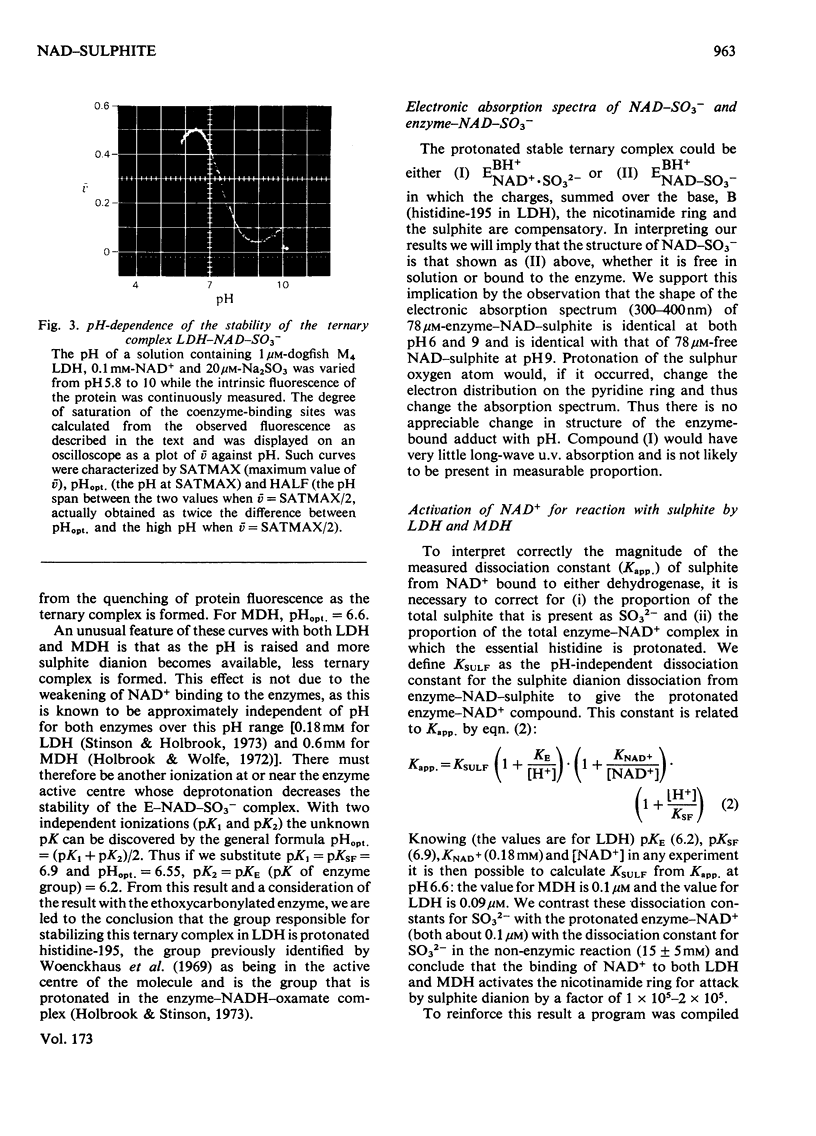
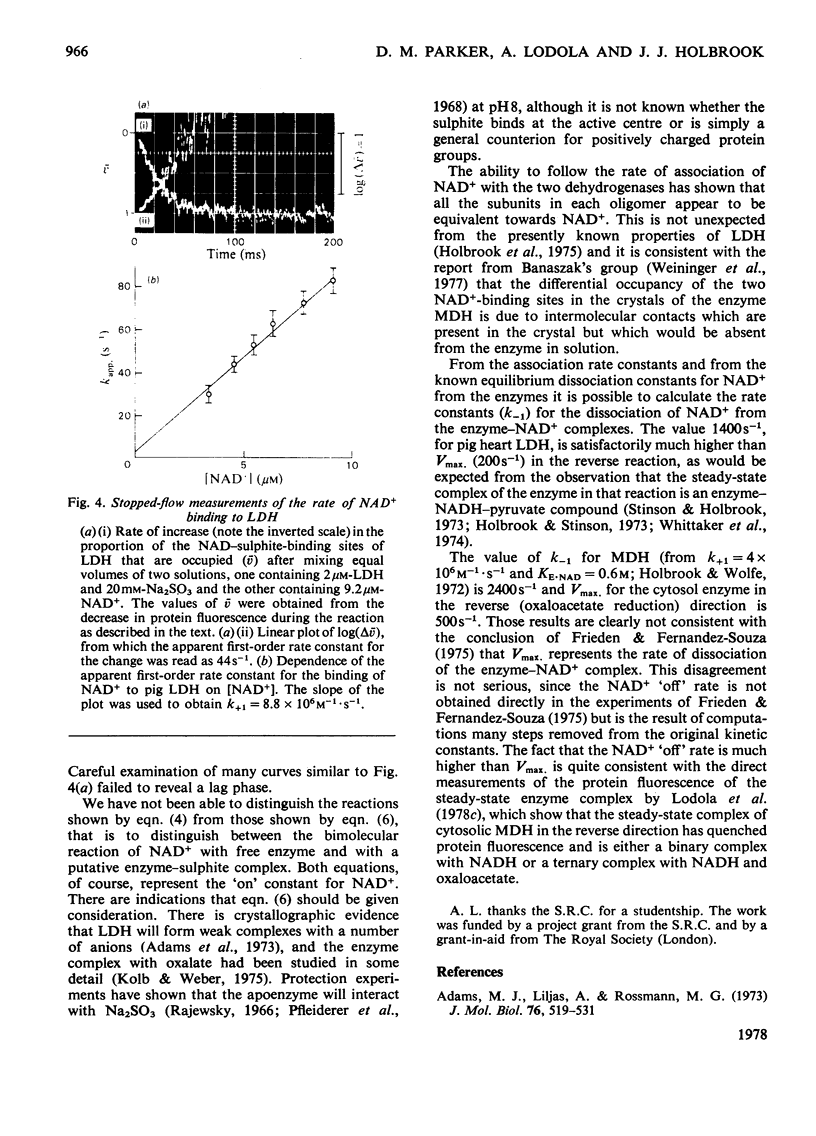
1. The formation of the non-enzymic adduct of NAD+ and sulphite was investigated. In agreement with others we conclude that the dianion of sulphite adds to NAD+. 2. The formation of ternary complexes of either lactate dehydrogenase or malate dehydrogenase with NAD+ and sulphite was investigated. The u.v. spectrum of the NAD–sulphite adduct was the same whether free or enzyme-bound at either pH6 or pH8. This suggests that the free and enzyme-bound adducts have a similar electronic structure. 3. The effect of pH on the concentration of NAD–sulphite bound to both enzymes was measured in a new titration apparatus. Unlike the non-enzymic adduct (where the stability change with pH simply reflects HSO3−=SO32−+H+), the enzyme-bound adduct showed a bell-shaped pH–stability curve, which indicated that an enzyme side chain of pK=6.2 must be protonated for the complex to form. Since the adduct does not bind to the enzyme when histidine-195 of lactate dehydrogenase is ethoxycarbonylated we conclude that the protein group involved is histidine-195. 4. The pH-dependence of the formation of a ternary complex of lactate dehydrogenase, NAD+ and oxalate suggested that an enzyme group is protonated when this complex forms. 5. The rate at which NAD+ binds to lactate dehydrogenase and malate dehydrogenase was measured by trapping the enzyme-bound NAD+ by rapid reaction with sulphite. The rate of NAD+ dissociation from the enzymes was calculated from the bimolecular association kinetic constant and from the equilibrium binding constant and was in both cases much faster than the forward Vmax.. No kinetic evidence was found that suggested that there were interactions between protein subunits on binding NAD+.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Liljas A., Rossman M. G. Functional anion binding sites in dogfish M4 lactate dehydrogenase. J Mol Biol. 1973 Jun 5;76(4):519–528. doi: 10.1016/0022-2836(73)90489-0. [DOI] [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- Ciaccio E. I. The inhibition of lactate dehydrogenase by 3-acetylpyridine adenine dinucleotide and bisulfite. J Biol Chem. 1966 Apr 10;241(7):1581–1586. [PubMed] [Google Scholar]
- Frieden C., Fernandez-Sousa J. Kinetic studies on pig heart cytoplasmic malate dehydrogenase. J Biol Chem. 1975 Mar 25;250(6):2106–2113. [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J. Protein fluorescence of lactate dehydrogenase. Biochem J. 1972 Jul;128(4):921–931. doi: 10.1042/bj1280921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. The use of ternary complexes to study ionizations and isomerizations during catalysis by lactate dehydrogenase. Biochem J. 1973 Apr;131(4):739–748. doi: 10.1042/bj1310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Wolfe R. G. Malate dehydrogenase. X. Fluorescence microtitration studies of D-malate, hydroxymalonate, nicotinamide dinucleotide, and dihydronicotinamide-adenine dinucleotide binding by mitochondrial and supernatant porcine heart enzymes. Biochemistry. 1972 Jun 20;11(13):2499–2502. doi: 10.1021/bi00763a018. [DOI] [PubMed] [Google Scholar]
- JECKEL D., PFLEIDERER G., WIELAND T. Uber die Einwirkung von Sulfit auf einige DPN hydrierende Enzyme. Biochem Z. 1956;328(3):187–194. [PubMed] [Google Scholar]
- JECSAI G. Crystalline lactic dehydrogenase from pig skeletal muscle. Acta Physiol Acad Sci Hung. 1961;20:339–346. [PubMed] [Google Scholar]
- Johnson S. L., Smith K. W. The interaction of borate and sulfite with pyridine nucleotides. Biochemistry. 1976 Feb 10;15(3):553–559. doi: 10.1021/bi00648a015. [DOI] [PubMed] [Google Scholar]
- Klinman J. P. Acid-base catalysis in the yeast alcohol dehydrogenase reaction. J Biol Chem. 1975 Apr 10;250(7):2569–2573. [PubMed] [Google Scholar]
- Kolb D. A., Weber G. Quantitative demonstration of the reciprocity of ligand effects in the ternary complex of chicken heart lactate dehydrogenase with nicotinamide adenine dinucleotide oxalate. Biochemistry. 1975 Oct 7;14(20):4471–4476. doi: 10.1021/bi00691a020. [DOI] [PubMed] [Google Scholar]
- Lodola A., Parker D. M., Jeck R., Holbrook J. J. Malate dehydrogenase of the cytosol. Ionizations of the enzyme-reduced-coenzyme complex and a comparison with lactate dehydrogenase. Biochem J. 1978 Aug 1;173(2):597–605. doi: 10.1042/bj1730597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodola A., Spragg S. P., Holbrook J. J. Malate dehydrogenase of the cytosol. Preparation and reduced nicotinamide-adenine dinucleotide-binding studies. Biochem J. 1978 Mar 1;169(3):577–588. doi: 10.1042/bj1690577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVOA W. B., SCHWERT G. W. Lactic dehydrogenase. VIII. Binding of oxamate and of oxalate by enzyme-coenzyme complexes. J Biol Chem. 1961 Jul;236:2150–2153. [PubMed] [Google Scholar]
- Pfleiderer G., Holbrook J. J., Zaki L., Jeckel D. The reactivities of the lysine, cysteine and tyrosine residues of pig heart lactate dehydrogenase in the presence of sulphite. FEBS Lett. 1968 Aug;1(3):129–132. doi: 10.1016/0014-5793(68)80039-0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Kreuzreagierende antigene Determinanten auf Lactatdehydrogenasen I und V durch Acetylierung. Biochim Biophys Acta. 1966 May 26;121(1):51–68. [PubMed] [Google Scholar]
- Shore J. D., Gilleland M. J. Binding and kinetic studies of liver alcohol dehydrogenase-coenzyme-pyrazole complexes. J Biol Chem. 1970 Jul 10;245(13):3422–3425. [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woenckhaus C., Berghäuser J., Pfleiderer G. Markierung essentieller Aminosäurereste der Lactat-Dehydrogenase aus Schweineherz mit (Carbonyl-14C)3-(2-Brom-acetyl)-pyridin. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):473–483. [PubMed] [Google Scholar]
- Woenckhaus C. Synthesis and properties of some new NAD analogues. Top Curr Chem. 1974;52:209–233. doi: 10.1007/3-540-06873-2_18. [DOI] [PubMed] [Google Scholar]