Abstract
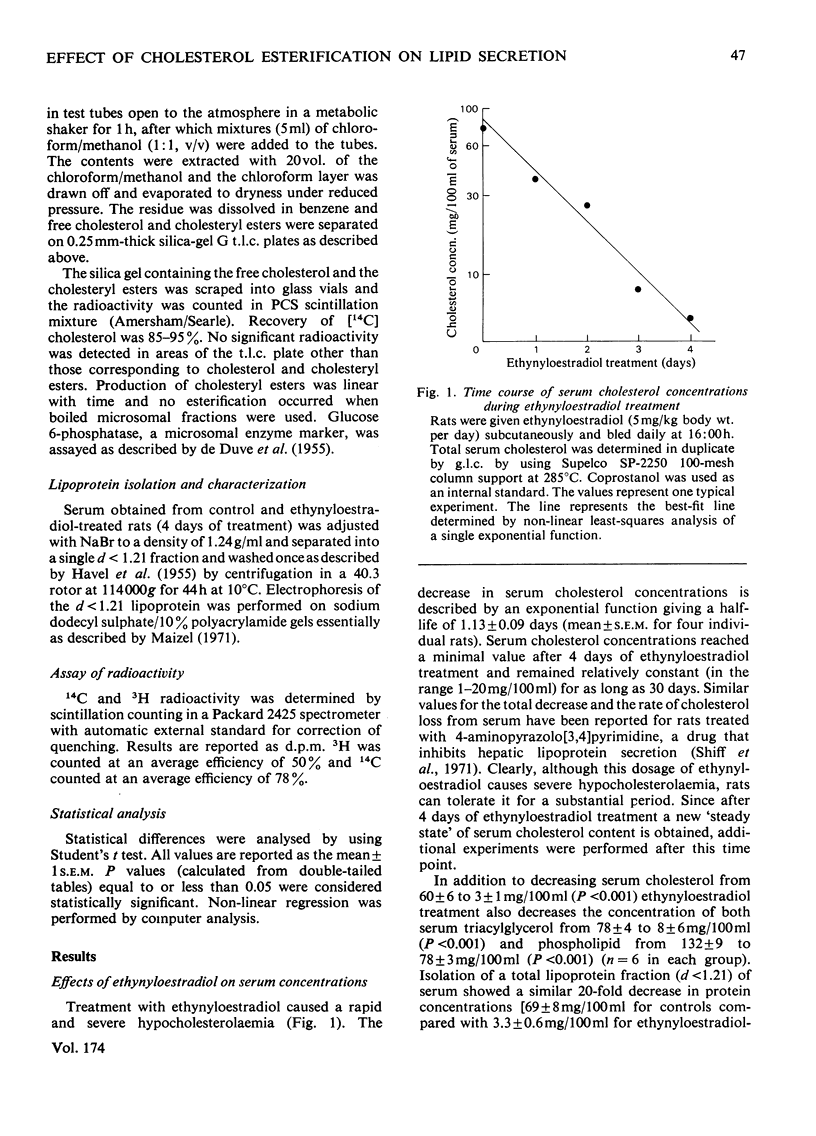
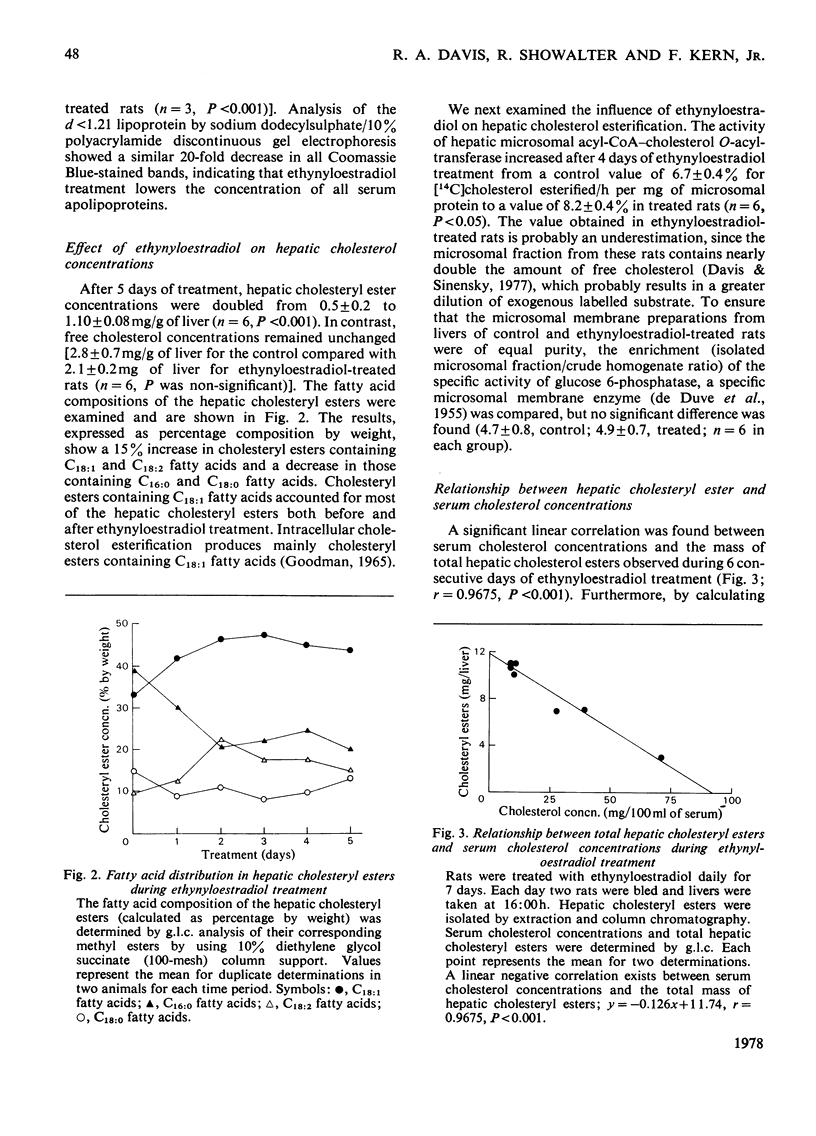
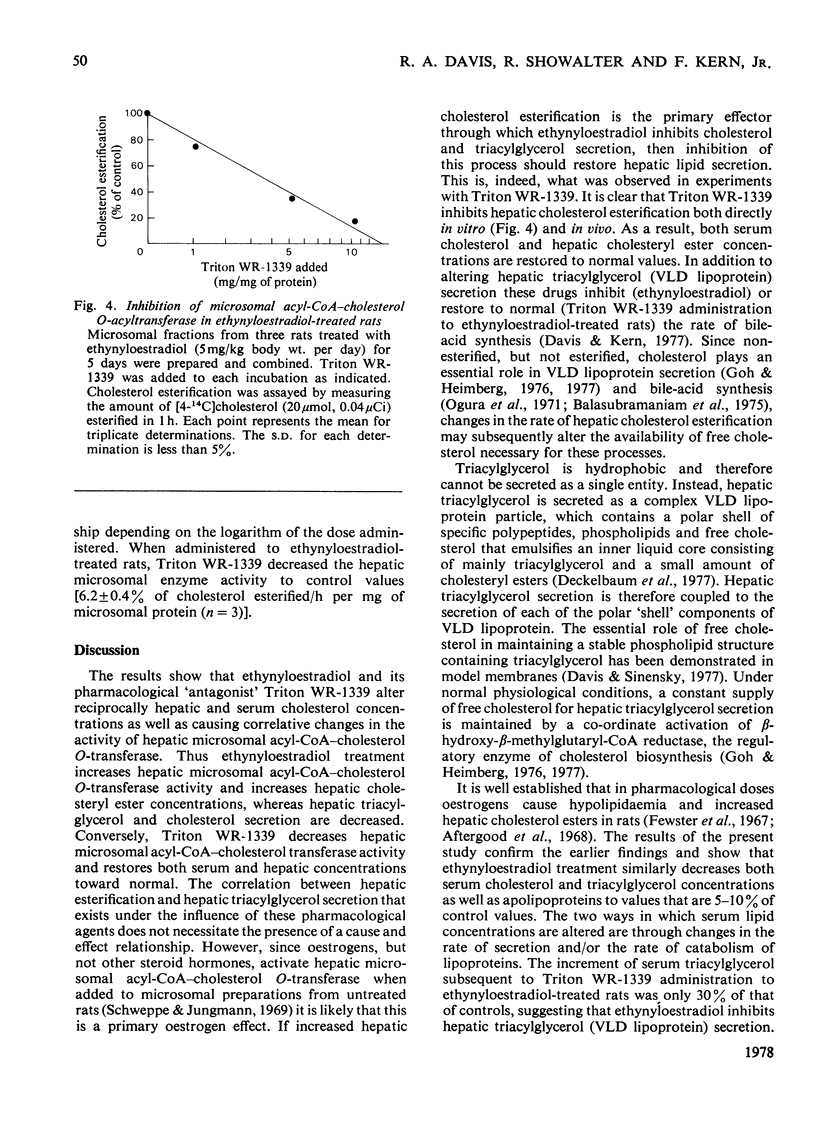
Rats treated with ethynyloestradiol have marked hypolipidaemia: serum cholesterol is decreased to 5%, triacylglycerol to 10% and phospholipid to 70% of control concentrations. Loss of serum cholesterol follows an exponential decay, with a half-life of 1.13±0.09 days. After 4 days of treatment, serum cholesterol concentrations remain relatively constant (ranging from 1 to 20mg/100ml) for at least 30 days. There is a concomitant 20-fold decrease in the d<1.21 fraction of serum proteins and a similar decrease in serum apolipoproteins as measured by sodium dodecyl sulphate/10%-polyacrylamide-gel electrophoresis. The activity of hepatic microsomal acyl-CoA–cholesterol O-acetyltransferase (EC 2.3.1.26) was significantly increased by ethynyloestradiol treatment (P<0.05). This activation caused hepatic cholesteryl esters containing mainly C18:1 fatty acids to increase linearly as serum cholesterol concentrations decreased (r=0.9675, P<0.001). Triton WR-1339, a non-ionic detergent that inhibits lipoprotein catabolism, was used to estimate hepatic lipid secretion by measuring the increment in serum lipids after its administration. At 15h after Triton WR-1339 administration, serum cholesterol concentrations were increased equally in both control and ethynyloestradiol-treated rats. In contrast, the increment of serum triacylglycerol of treated rats was 40% of that found in control rats, indicating that ethynyloestradiol inhibits hepatic triacylglycerol secretion. Triton WR-1339 inhibited the oestrogen activation of hepatic microsomal acyl-CoA–cholesterol O-acyltransferase and restored hepatic cholesteryl ester concentrations to normal values. These data suggest that ethynyloestradiol and its pharmacological `antagonist' Triton WR-1339 alter hepatic triacylglycerol secretion via a mechanism associated with changes in hepatic cholesterol esterification.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftergood L., Hernandez H. J., Alfin-Slater R. B. Effect of large doses of the oral contraceptive Enovid on cholesterol metabolism in the rat. J Lipid Res. 1968 Jul;9(4):447–452. [PubMed] [Google Scholar]
- BYERS S. O., FIEDMAN M., SUGIYAMA T. Triton hypercholesteremia: cause or consequence of augmented cholesterol synthesis. Am J Physiol. 1963 Jun;204:1100–1102. doi: 10.1152/ajplegacy.1963.204.6.1100. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Kern F., Jr Effects of ethinyl estradiol and phenobarbital on bile acid synthesis and biliary bile acid and cholesterol excretion. Gastroenterology. 1976 Jun;70(6):1130–1135. [PubMed] [Google Scholar]
- Deckelbaum R. J., Tall A. R., Small D. M. Interaction of cholesterol ester and triglyceride in human plasma very low density lipoprotein. J Lipid Res. 1977 Mar;18(2):164–168. [PubMed] [Google Scholar]
- Fewster M. E., Pirrie R. E., Turner D. A. Effect of estradiol benzoate on lipid metabolism in the rat. Endocrinology. 1967 Feb;80(2):263–271. doi: 10.1210/endo-80-2-263. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S., DEYKIN D., SHIRATORI T. THE FORMATION OF CHOLESTEROL ESTERS WITH RAT LIVER ENZYMES. J Biol Chem. 1964 May;239:1335–1345. [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Effect of oleic acid and cholesterol on the activity of hepatic hydroxymethylglutaryl coenzyme A reductase. FEBS Lett. 1976 Mar 15;63(1):209–210. doi: 10.1016/0014-5793(76)80228-1. [DOI] [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Effects of free fatty acids on activity of hepatic microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase and on secretion of triglyceride and cholesterol by liver. J Biol Chem. 1977 May 10;252(9):2822–2826. [PubMed] [Google Scholar]
- Goodman D. S. Cholesterol ester metabolism. Physiol Rev. 1965 Oct;45(4):747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. V., Pottenger L. A., Reingold A. L., Getz G. S., Wissler R. W. Degradation of I 125 -labelled serum low density lipoprotein in normal and estrogen-treated male rats. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1471–1477. doi: 10.1016/s0006-291x(71)80251-6. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauda H. C., Zilversmit D. B. Influx of cholesterol into plasma in rabbits with fasting hyperbetalipoproteinemia. J Lipid Res. 1974 Nov;15(6):593–601. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackinnon M., Simon F. Pharmacological reversal of cholestasis-associated decrease in hepatic cytochrome P-450. Biochem Pharmacol. 1975 Mar 15;24(6):748–749. doi: 10.1016/0006-2952(75)90256-7. [DOI] [PubMed] [Google Scholar]
- Mahaffee D., Reitz R. C., Ney R. L. The mechanism of action of adrenocroticotropic hormone. The role of mitochondrial cholesterol accumulation in the regulation of steroidogenesis. J Biol Chem. 1974 Jan 10;249(1):227–233. [PubMed] [Google Scholar]
- ORMOND A. P., Jr, RIVERA-VELEZ J. M. BLOOD VOLUME IN RELATION TO BODY WEIGHT OF THE MALE RAT USING RADIO-IODINATED SERUM ALBUMIN. Proc Soc Exp Biol Med. 1965 Mar;118:600–602. doi: 10.3181/00379727-118-29915. [DOI] [PubMed] [Google Scholar]
- Ogura M., Shiga J., Yamasaki K. Studies on the cholesterol pool as the precursor of bile acids in the rat. J Biochem. 1971 Dec;70(6):967–972. doi: 10.1093/oxfordjournals.jbchem.a129726. [DOI] [PubMed] [Google Scholar]
- Schweppe J. S., Jungmann R. A. The effect of hormones on hepatic cholesterol ester synthesis in vitro. Proc Soc Exp Biol Med. 1969 Jul;131(3):868–870. doi: 10.3181/00379727-131-33997. [DOI] [PubMed] [Google Scholar]
- Shiff T. S., Roheim P. S., Eder H. A. Effects of high sucrose diets and 4-aminopyrazolopyrimidine on serum lipids and lipoproteins in the rat. J Lipid Res. 1971 Sep;12(5):596–603. [PubMed] [Google Scholar]
- Simon F. R., Arias I. M. Alteration of bile canalicular enzymes in cholestasis. A possible cause of bile secretory failure. J Clin Invest. 1973 Apr;52(4):765–775. doi: 10.1172/JCI107239. [DOI] [PMC free article] [PubMed] [Google Scholar]


