Abstract
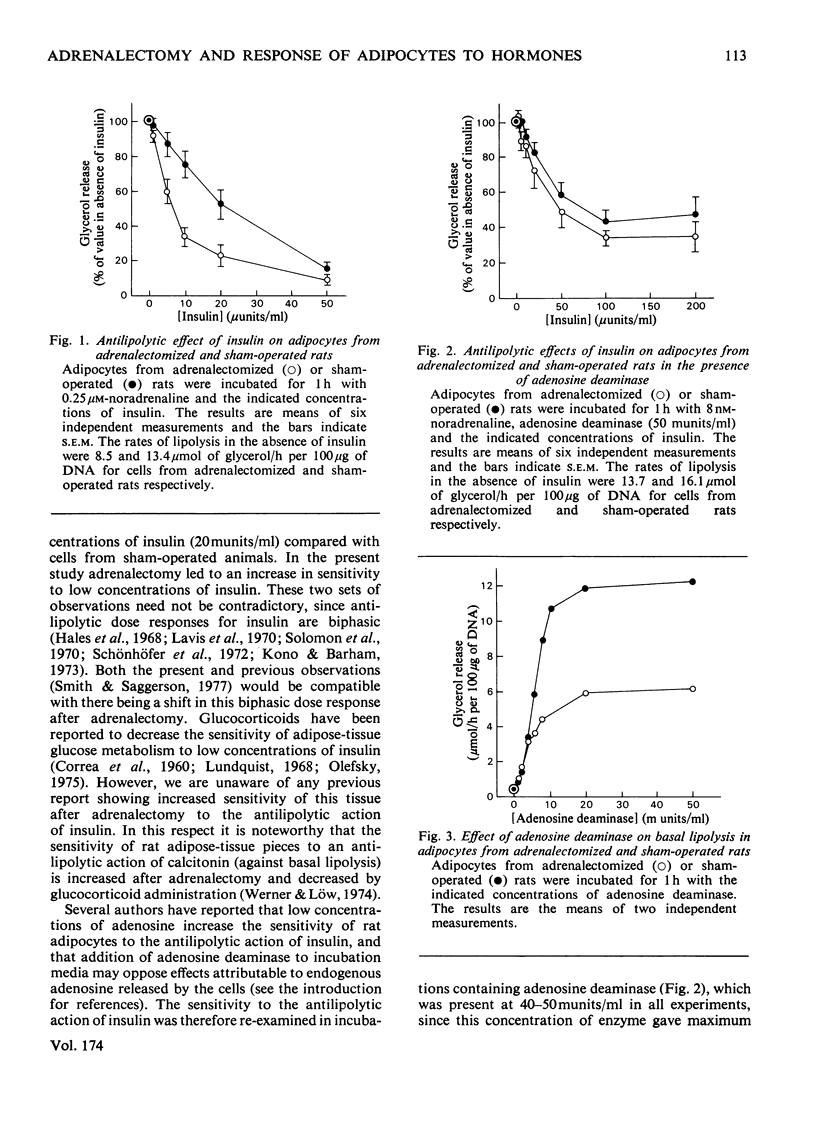
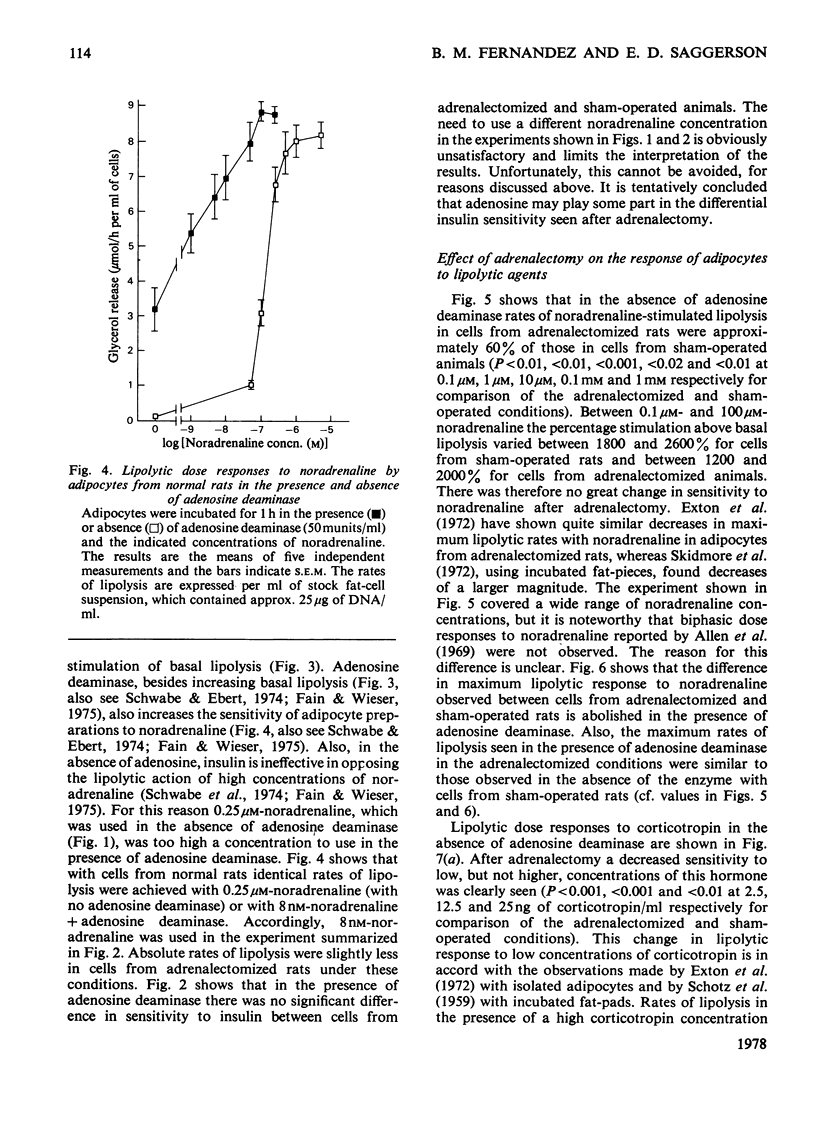
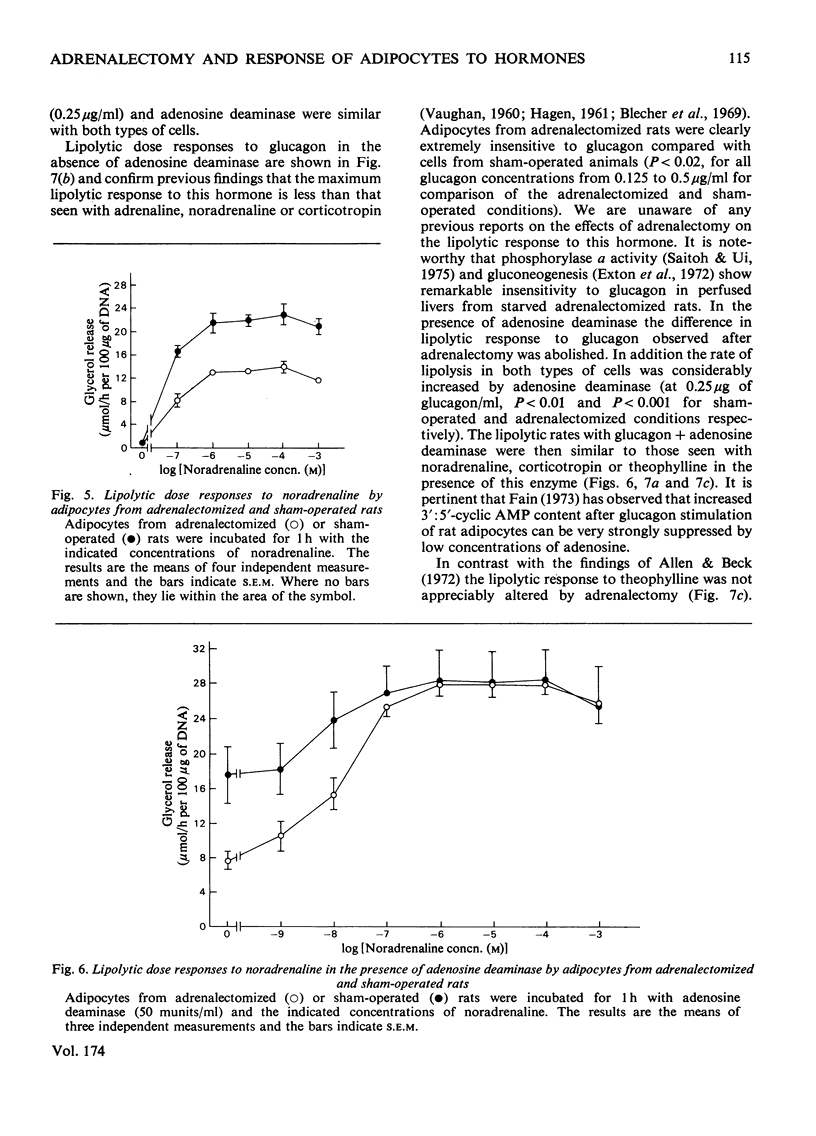
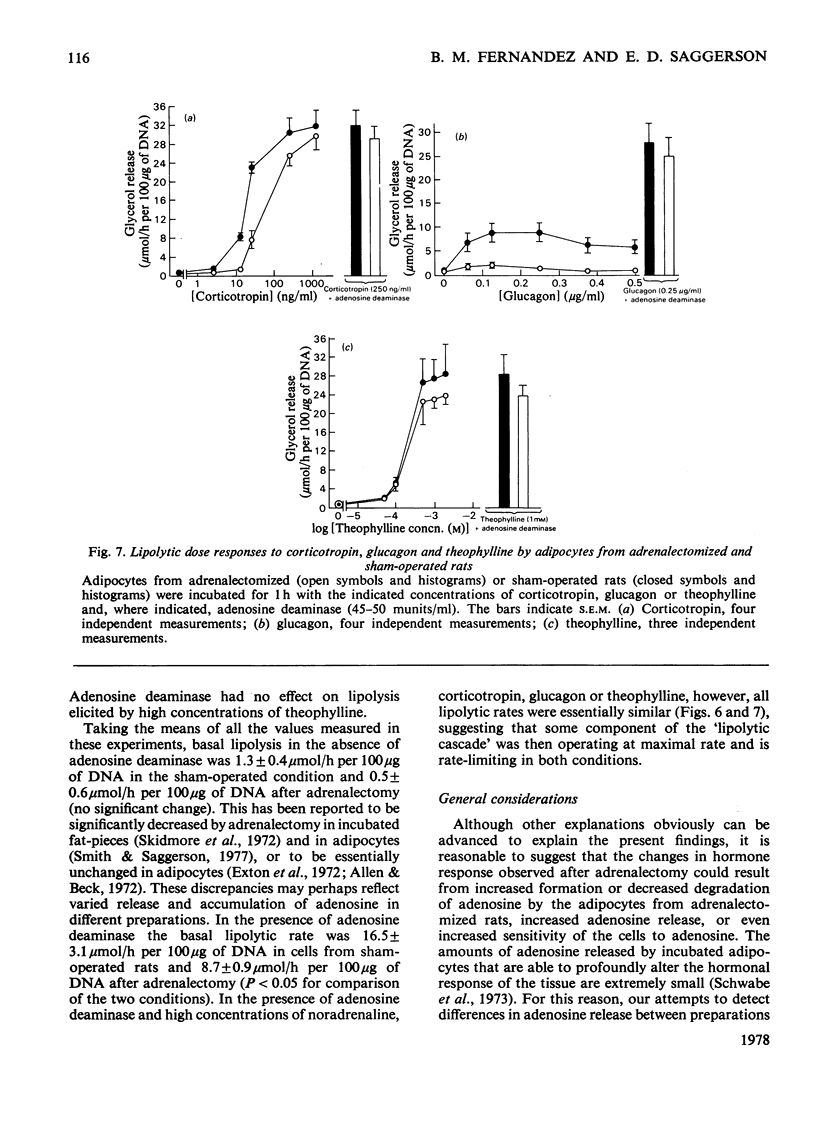
1. Adipocytes isolated from rats 6--9 days after adrenalectomy had significantly increased sensitivity to insulin action against noradrenaline-stimulated lipolysis. In the presence of adenosine deaminase there was no significant difference in insulin sensitivity between cells from adrenalectomized and sham-operated rats. 2. Adipocytes from adrenalectomized rats had decreased lipolytic responses to all concentrations of noradrenaline and glucagon tested and a decreased lipolytic response to low but not high concentrations of corticotropin. There was no difference in lipolytic response to theophylline after adrenalectomy. Adenosine deaminase corrected the differences in response to noradrenaline and glucagon resulting from adrenalectomy. 3. In the presence of adenosine deaminase rates of lipolysis, after stimulation by high concentrations of noradrenaline, glucagon, corticotropin or theophylline, were the same in cells from adrenalectomized or sham-operated rats. 4. These findings and previously reported effects of adenosine and adrenalectomy on adipocyte function are discussed. It is proposed that changes in adipocyte hormone responsiveness after adrenalectomy may result from changes in adenosine metabolism or release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afonso S., O'Brien G. S. Inhibition of cardiovascular metabolic and hemodynamic effects of adenosine by aminophylline. Am J Physiol. 1970 Dec;219(6):1672–1674. doi: 10.1152/ajplegacy.1970.219.6.1672. [DOI] [PubMed] [Google Scholar]
- Allen D. O., Beck R. R. Alterations in lipolysis, adenylate cyclase and adenosine 3', 5'-monophosphate levels in isolated fat cells following adrenalectomy. Endocrinology. 1972 Aug;91(2):504–510. doi: 10.1210/endo-91-2-504. [DOI] [PubMed] [Google Scholar]
- Allen D. O., Hillman C. C., Ashmore J. Studies on a biphasic lipolytic response to catecholamines in isolated fat cells. Biochem Pharmacol. 1969 Sep;18(9):2233–2240. doi: 10.1016/0006-2952(69)90330-x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Hormone-sensitive adenylyl cyclases. Useful models for studying hormone receptor functions in cell-free systems. Biochim Biophys Acta. 1973 Sep 10;300(2):129–158. doi: 10.1016/0304-4157(73)90002-6. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Cameron T. Influence of nucleotide derivatives on lipolysis and lipid peroxide formation in vitro. Metabolism. 1967 Jan;16(1):91–95. doi: 10.1016/0026-0495(67)90164-3. [DOI] [PubMed] [Google Scholar]
- Blecher M., Merlino N. S., Ro'Ane J. T., Flynn P. D. Independence of the effects of epinephrine, glucagon, and adrenocorticotropin on glucose utilization from those on lipolysis in isolated rat adipose cells. J Biol Chem. 1969 Jul 10;244(13):3423–3429. [PubMed] [Google Scholar]
- CORREA P. R., MAGALHAES E., KRAHL M. E. Response of epididymal adipose tissue to small concentrations of insulin: effect of cortisone. Proc Soc Exp Biol Med. 1960 Apr;103:704–706. doi: 10.3181/00379727-103-25641. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Briones R., Piña E. Inhibition by adenosine of the cortisol-induced liver glycogen accumulation in adrenalectomized rats. Biochem Pharmacol. 1971 Oct;20(10):2535–2541. doi: 10.1016/0006-2952(71)90161-4. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DOLE V. P. Effect of nucleic acid metabolites on lipolysis in adipose tissue. J Biol Chem. 1961 Dec;236:3125–3130. [PubMed] [Google Scholar]
- DOLE V. P. Insulin-like actions of ribonucleic acid, adenylic acid, and adenosine. J Biol Chem. 1962 Sep;237:2758–2762. [PubMed] [Google Scholar]
- Davies J. I. In vitro regulation of the lipolysis of adipose tissue. Nature. 1968 Apr 27;218(5139):349–352. doi: 10.1038/218349a0. [DOI] [PubMed] [Google Scholar]
- Ebert R., Schwabe U. Studies on the antilipolytic effect of adenosine and related compounds in isolated fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):247–259. doi: 10.1007/BF00500286. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Friedmann N., Wong E. H., Brineaux J. P., Corbin J. D., Park C. R. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J Biol Chem. 1972 Jun 10;247(11):3579–3588. [PubMed] [Google Scholar]
- Fain J. N. Inhibition of adenosine cyclic 3', 5'-monophosphate accumulation in fat cells by adenosine, N6-(phenylisopropyl) adenosine, and related compounds. Mol Pharmacol. 1973 Sep;9(5):595–604. [PubMed] [Google Scholar]
- Fain J. N., Pointer R. H., Ward W. F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972 Nov 10;247(21):6866–6872. [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF VARIOUS DRUGS ON CALCIUM EXCHANGE IN THE ISOLATED GUINEA-PIG LEFT AURICLE. J Pharmacol Exp Ther. 1964 Aug;145:162–172. [PubMed] [Google Scholar]
- Gerlach A., van Zwieten P. A. Mechanical performance and calcium metabolism in rat isolated heart muscle after adrenalectomy. Pflugers Arch. 1969;311(1):96–108. doi: 10.1007/BF00588064. [DOI] [PubMed] [Google Scholar]
- Guthrie J. R., Nayler W. G. Interaction between caffeine and adenosine on calcium exchangeability in mammalian atria. Arch Int Pharmacodyn Ther. 1967 Nov;170(1):249–255. [PubMed] [Google Scholar]
- HAGEN J. H. Effect of glucagon on the metabolism of adipose tissue. J Biol Chem. 1961 Apr;236:1023–1027. [PubMed] [Google Scholar]
- Kappeler H. Zur Pharmakologie der Lipolysehemmung. I. Wirkungsweise adenosinhaltiger Nucleoside und Nucleotide auf die Lipolyse des Fettgewebes in vitro. Diabetologia. 1966 Jun;2(1):52–61. doi: 10.1007/BF01106974. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Effects of insulin on the levels of adenosine 3':5'-monophosphate and lipolysis in isolated rat epididymal fat cells. J Biol Chem. 1973 Nov 10;248(21):7417–7426. [PubMed] [Google Scholar]
- LEBOEUF B., RENOLD A. E., CAHILL G. F., Jr Studies on rat adipose tissue in vitro. IX. Further effects cortisol on glucose metabolism. J Biol Chem. 1962 Apr;237:988–991. [PubMed] [Google Scholar]
- Livingston J. N., Lockwood D. H. Effect of glucocorticoids on the glucose transport system of isolated fat cells. J Biol Chem. 1975 Nov 10;250(21):8353–8360. [PubMed] [Google Scholar]
- Lundquist I. On the significance of serum dilution and cortisol antagonism in the rat fat pad bioassay of insulin. Acta Endocrinol (Copenh) 1968 May;58(1):11–26. doi: 10.1530/acta.0.0580011. [DOI] [PubMed] [Google Scholar]
- MUNCK A. Studies on the mode of action of glucocorticoids in rats. II. The effects in vivo and in vitro on net glucose uptake by isolated adipose tissue. Biochim Biophys Acta. 1962 Feb 26;57:318–326. doi: 10.1016/0006-3002(62)91125-3. [DOI] [PubMed] [Google Scholar]
- Maickel R. P., Stern D. N., Takabatake E., Brodie B. B. The sympathetic nervous system as a homeostatic mechanism. II. Effect of adrenocortical hormones on body temperature maintenance of cold-exposed adrenalectomized rats. J Pharmacol Exp Ther. 1967 Jul;157(1):111–116. [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpeira J. N., Holland G. F. The effect of nicotinamide adenine dinucleotide on lipolysis in adipose tissue in vitro. Experientia. 1966 Oct 15;22(10):658–659. doi: 10.1007/BF01902426. [DOI] [PubMed] [Google Scholar]
- RESHEF L., SHAPIRO B. Effect of epinephrine, cortisone and growth hormone on release of unesterified fatty acids by adipose tissue in vitro. Metabolism. 1960 Jun;9:551–555. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rovetto M. J., Lefer A. M. Electrophysiologic properties of cardiac muscle in adrenal insufficiency. Am J Physiol. 1970 Apr;218(4):1015–1019. doi: 10.1152/ajplegacy.1970.218.4.1015. [DOI] [PubMed] [Google Scholar]
- SCHOTZ M. C., MASSON G. M., PAGE I. H. ACTH in vitro on release of nonesterified fatty acids from adipose tissues of adrenalectomized rats. Proc Soc Exp Biol Med. 1959 May;101(1):159–161. doi: 10.3181/00379727-101-24866. [DOI] [PubMed] [Google Scholar]
- SHAFRIR E., KERPEL S. FATTY ACID ESTERIFICATION AND RELEASE AS RELATED TO THE CARBOHYDRATE METABOLISM OF ADIPOSE TISSUE: EFFECT OF EPINEPHRINE, CORTISOL, AND ADRENALECTOMY. Arch Biochem Biophys. 1964 May;105:237–246. doi: 10.1016/0003-9861(64)90004-9. [DOI] [PubMed] [Google Scholar]
- SHAFRIR E., SUSSMAN K. E., STEINBERG D. Role of the pituitary and the adrenal in the mobilization of free fatty acids and lipoproteins. J Lipid Res. 1960 Oct;1:459–465. [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y., Ui M. Activation and inactivation of phosphorylase and glycogen synthetase during perfusion of rat liver as influenced by epinephrine, glucagon and hydrocortisone. Biochim Biophys Acta. 1975 Sep 8;404(1):7–17. doi: 10.1016/0304-4165(75)90142-7. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from fat cells: effect on cyclic AMP levels and hormone actions. Adv Cyclic Nucleotide Res. 1975;5:569–584. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R. Stimulation of cyclic adenosine 3',5'-monophosphate accumulation and lipolysis in fat cells by adenosine deaminase. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):33–44. doi: 10.1007/BF00647401. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Schönhöfer P. S., Ebert R. Facilitation by adenosine of the action of insulin on the accumulation of adenosine 3':5'-monophosphate, lipolysis, and glucose oxidation in isolated fat cells. Eur J Biochem. 1974 Aug 1;46(3):537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Schönhöfer P. S., Skidmore I. F., Paul M. I., Ditzion B. R., Pauk G. L., Krishna G. Effects of glucocorticoids on adenyl cyclase and phosphodiesterase activity in fat cell homogenates and the accumulation of cyclic AMP in intact fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(3):267–282. doi: 10.1007/BF00501418. [DOI] [PubMed] [Google Scholar]
- Schönhöfer P., Skidmore I. F., Bourne H. R., Krishna G. A., Brodie B. B. Influence of adrenal cortical hormones on the norepinephrine-induced lipolysis. Arzneimittelforschung. 1968 Dec;18(12):1540–1541. [PubMed] [Google Scholar]
- Skidmore I. F., Schönhöfer P. S., Bourne H. R., Krishna G. Effect of adrenalectomy and cortisone replacement on the lipolysis in fat tissue and fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(2):113–124. doi: 10.1007/BF00501846. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Saggerson E. D. Decreased sensitivity to insulin in white adipose tissue from adrenalectomised rats. Horm Metab Res. 1977 Nov;9(6):474–480. doi: 10.1055/s-0028-1093503. [DOI] [PubMed] [Google Scholar]
- Solomon S. S., Brush J. S., Kitabchi A. E. Antilipolytic activity of insulin and proinsulin on ACTH and cyclic nucleotide-induced lipolysis in the isolated adipose cell of rat. Biochim Biophys Acta. 1970 Oct 6;218(1):167–169. doi: 10.1016/0005-2760(70)90104-9. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M. Effect of hormones on phosphorylase activity in adipose tissue. J Biol Chem. 1960 Nov;235:3049–3053. [PubMed] [Google Scholar]
- Werner S., Alm B., Löw H. Effects of adrenalectomy and resubstitution with moderate and high doses of cortisone acetate on lipolysis and 47 calcium uptake in rat adipose tissue in vivo. Horm Metab Res. 1972 May;4(3):195–201. doi: 10.1055/s-0028-1094048. [DOI] [PubMed] [Google Scholar]
- Werner S., Löw H. Inhibitory effects of calcitonin on lipolysis and 47 calcium accumulation in rat adipose tissue in vivo. Horm Metab Res. 1974 Jan;6(1):30–36. doi: 10.1055/s-0028-1093899. [DOI] [PubMed] [Google Scholar]
- Wieser P. B., Fain J. N. Insulin, prostaglandin E1, PHENYLISOPROPYLADENOSINE AND NICOTINIC ACID AS REGULATORS OF FAT CELL METABOLISM. Endocrinology. 1975 May;96(5):1221–1225. doi: 10.1210/endo-96-5-1221. [DOI] [PubMed] [Google Scholar]
- Yorke R. E. The influence of dexamethasone on adipose tissue metabolism in vitro. J Endocrinol. 1967 Nov;39(3):329–343. doi: 10.1677/joe.0.0390329. [DOI] [PubMed] [Google Scholar]


