Abstract
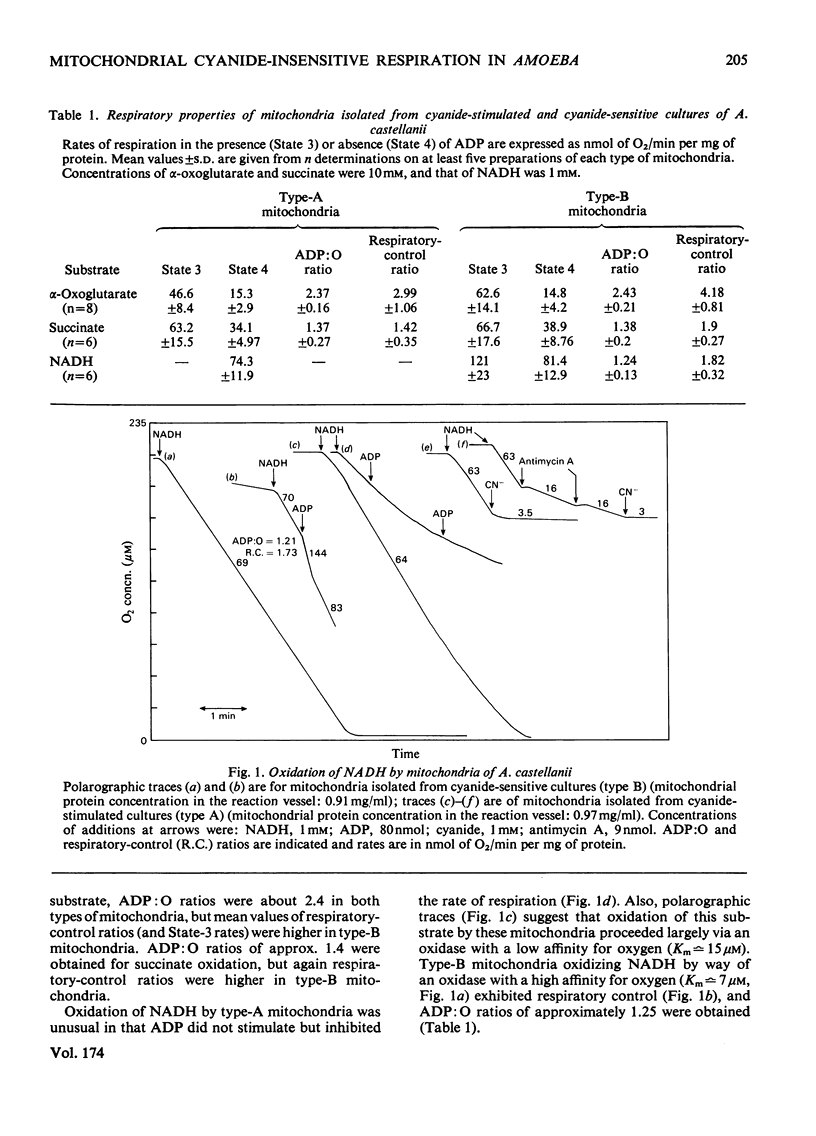
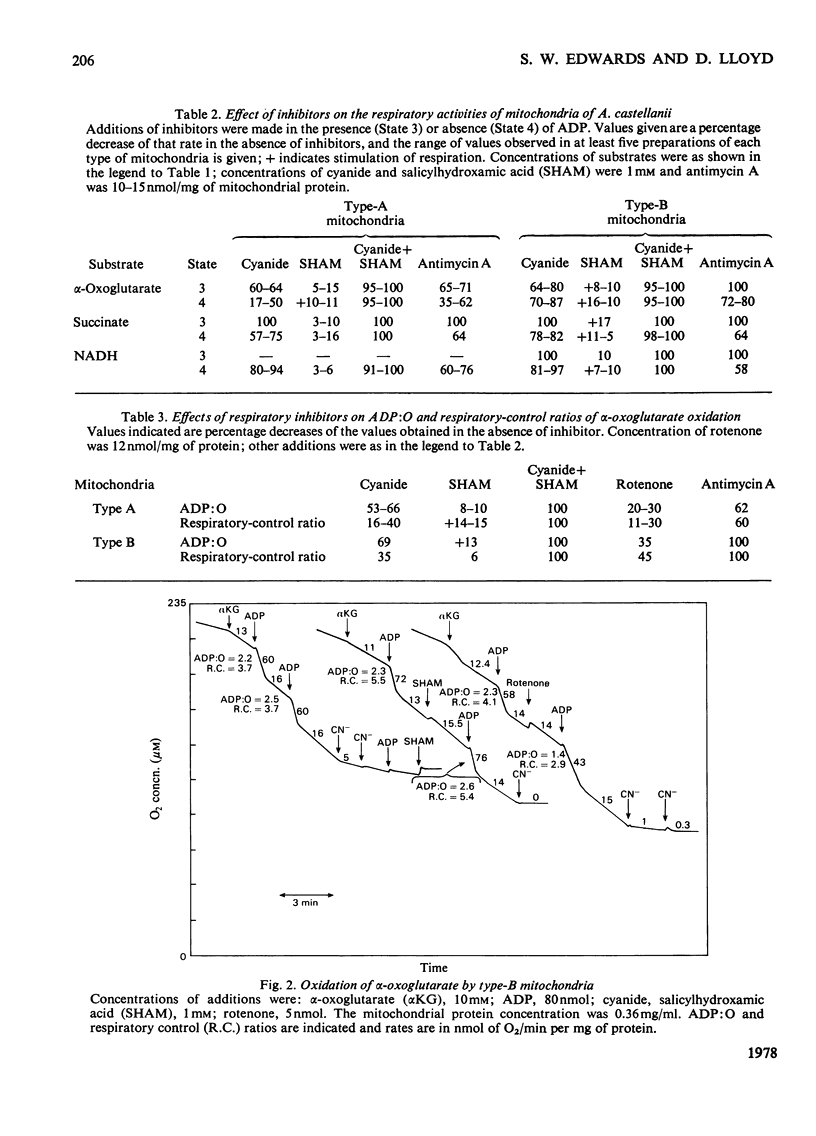
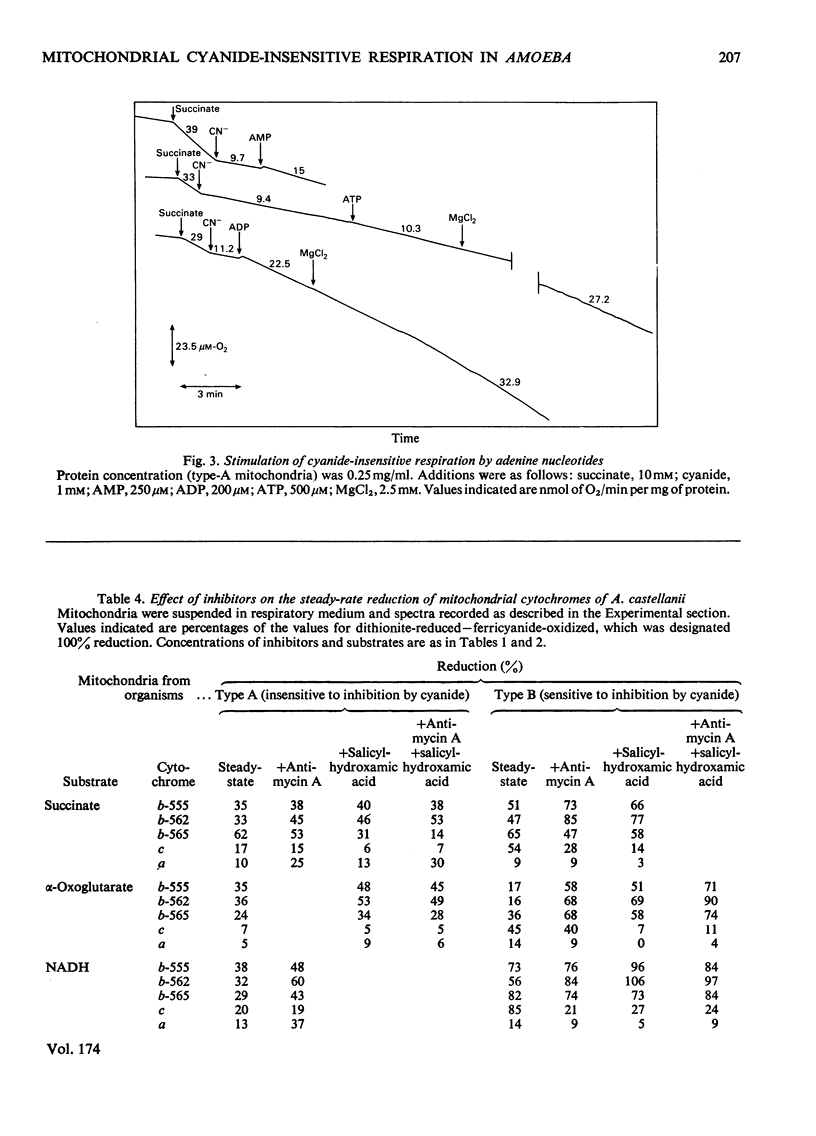
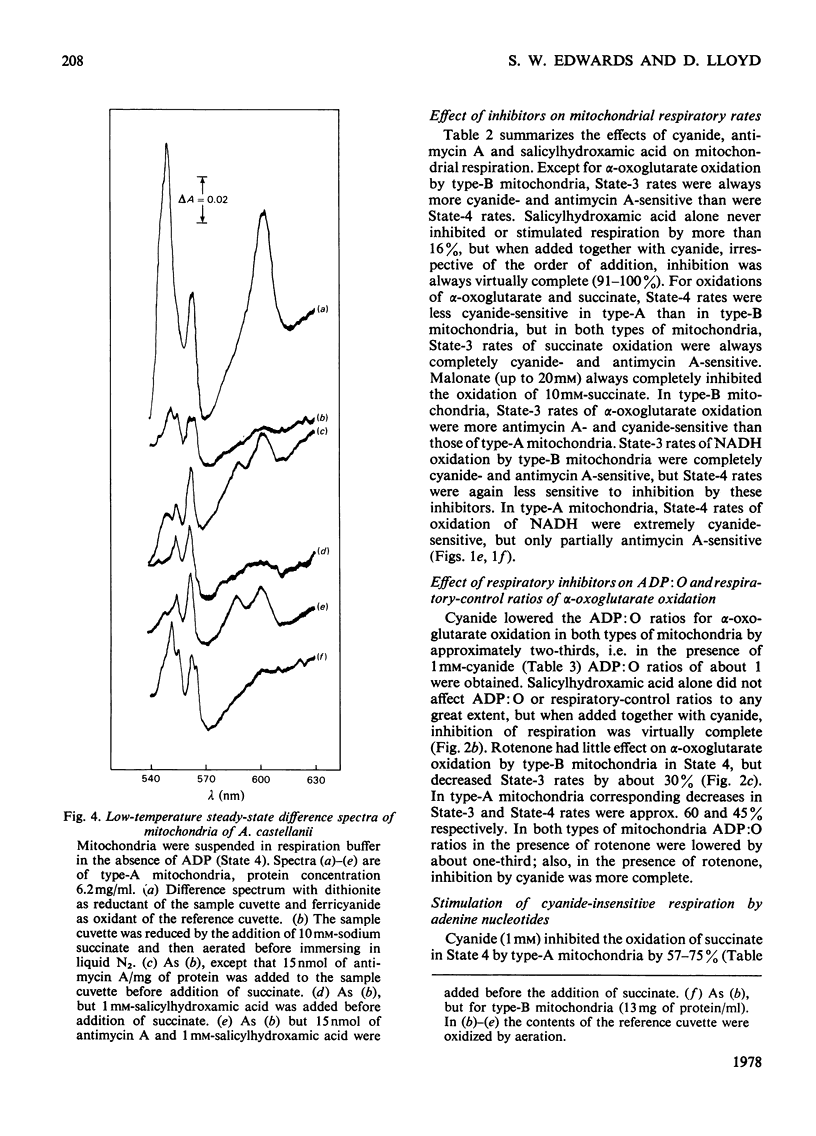
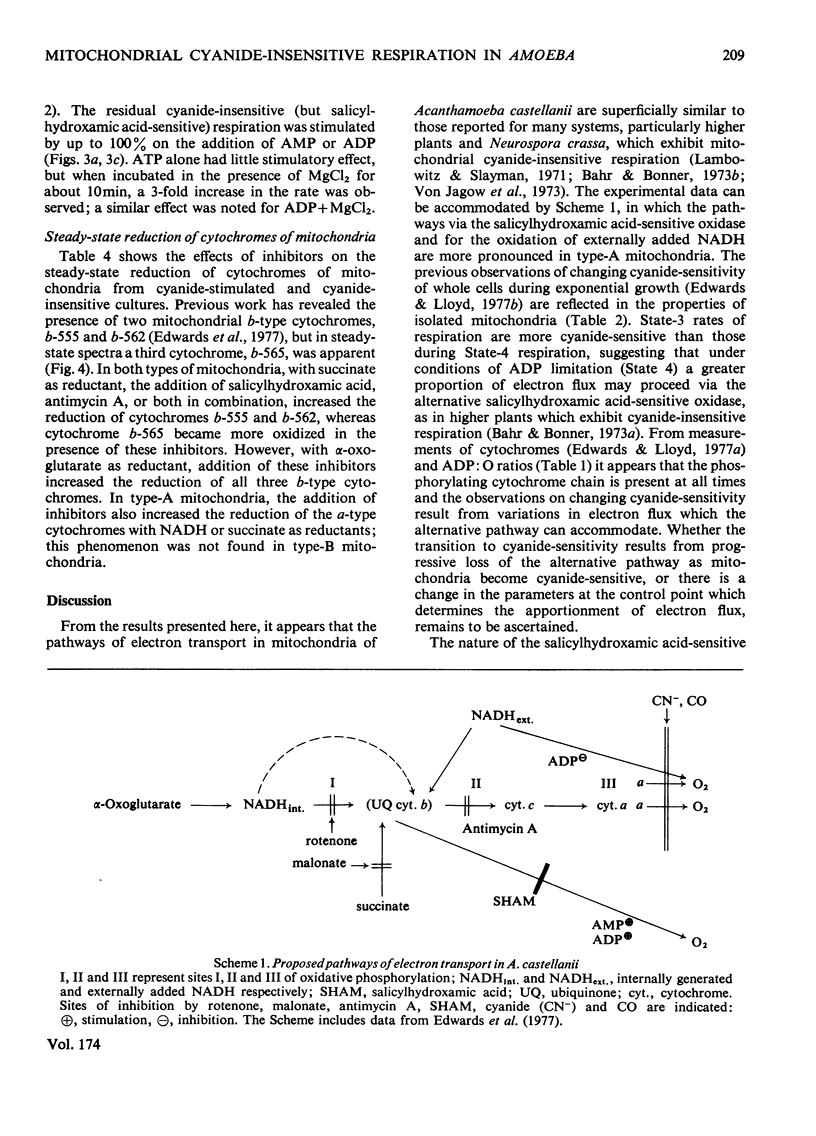
1. Mitochondria isolated from cultures of Acanthamoeba castellanii exhibit respiratory control and oxidize α-oxoglutarate, succinate and NADH with ADP:O ratios of about 2.4, 1.4 and 1.25 respectively. 2. Mitochondria from cultures of which the respiration was stimulated up to 50% by 1mm-cyanide (type-A mitochondria) and from cyanide-sensitive cultures (type-B mitochondria) had similar respiratory-control ratios and ADP:O ratios. 3. State-3 rates of respiration were generally more cyanide-sensitive than State-4 rates, and the respiration of type-A mitochondria was more cyanide-resistant than that of type-B mitochondria. 4. Salicylhydroxamic acid alone had little effect on respiratory activities of either type of mitochondria, but when added together with cyanide, irrespective of the order of addition, inhibition was almost complete. 5. Oxidation of externally added NADH by type-A mitochondria was mainly via an oxidase with a low affinity for oxygen (Km[unk]15μm), which was largely cyanide-sensitive and partially antimycin A-sensitive; this electron-transport pathway was inhibited by ADP. 6. Cyanide-insensitive but salicylhydroxamic acid-sensitive respiration was stimulated by AMP and ADP, and by ATP after incubation in the presence of MgCl2. 7. Addition of rotenone to mitochondria oxidizing α-oxoglutarate lowered the ADP:O ratios by about one-third and rendered inhibition by cyanide more complete. 8. The results suggest that mitochondria of A. castellanii possess branched pathways of electron transport which terminate in three separate oxidases; the proportions of electron fluxes via these pathways vary at different stages of growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B. The nature of electron transfer and energy coupling reactions. FEBS Lett. 1972 Jun 1;23(1):3–20. doi: 10.1016/0014-5793(72)80272-2. [DOI] [PubMed] [Google Scholar]
- Edwards C., Lloyd D. Terminal oxidases and carbon monoxide-reacting haemoproteins in the trypanosomatid, Crithidia fasciculata. J Gen Microbiol. 1973 Dec;79(2):275–284. doi: 10.1099/00221287-79-2-275. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Chagla A. H., Griffiths A. J., Lloyd D. The cytochromes of Acanthamoeba castellanii. Biochem J. 1977 Oct 15;168(1):113–121. doi: 10.1042/bj1680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A. Growth phase and the number of phosphorylation sites in the mitochondrial electron transport chain of Acanthamoeba castellanii. J Protozool. 1973 May;20(2):336–338. doi: 10.1111/j.1550-7408.1973.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens L., Verachtert H. Adenosine 5'-monophosphate-stimulated cyanide-insensitive respiration in mitochondria of Moniliella tomentosa. J Bacteriol. 1976 Mar;125(3):829–836. doi: 10.1128/jb.125.3.829-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Bonner W. D., Jr, Nyns E. J. Involvement of iron in the biogenesis of the cyanide-insensitive respiration in the yeast Saccharomycopsis lipolytica. Biochim Biophys Acta. 1977 Apr 11;460(1):94–100. doi: 10.1016/0005-2728(77)90155-4. [DOI] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Katz R. Growth phase and rotenone sensitivity in Torulopsis utilis: Difference between exponential and stationary phase. FEBS Lett. 1971 Jan 12;12(3):153–156. doi: 10.1016/0014-5793(71)80056-x. [DOI] [PubMed] [Google Scholar]
- Katz R., Kilpatrick L., Chance B. Acquisition and loss of rotenone sensitivity in Torulopsis utilis. Eur J Biochem. 1971 Aug 16;21(3):301–307. doi: 10.1111/j.1432-1033.1971.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Knowles C. J. Microorganisms and cyanide. Bacteriol Rev. 1976 Sep;40(3):652–680. doi: 10.1128/br.40.3.652-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D., Griffiths A. J. The isolation of mitochondria from the amoeba Hartmanella castellanii Neff. Exp Cell Res. 1968 Aug-Sep;51(2-3):291–300. doi: 10.1016/0014-4827(68)90122-5. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975 Nov 15;59(2):137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Rich P. R., Bonner W. D., Jr, Ingledew W. J. A complex EPR signal in mung bean mitochondria and its possible relation to the alternate pathway. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1099–1107. doi: 10.1016/s0006-291x(76)80245-8. [DOI] [PubMed] [Google Scholar]
- Ohnishi T. Factors controlling the occurrence of site I phosphorylation in C. utilis mitochondria. FEBS Lett. 1972 Aug 15;24(3):305–309. doi: 10.1016/0014-5793(72)80378-8. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jagow G., Weiss H., Klingenberg M. Comparison of the respiratory chain of Neurospora crassa wild type and the mi-mutants mi-1 and mi-3. Eur J Biochem. 1973 Feb 15;33(1):140–157. doi: 10.1111/j.1432-1033.1973.tb02665.x. [DOI] [PubMed] [Google Scholar]


