Abstract
Cells of Rhodopseudomonas spheroides, strains R-26 or GVP, were grown photosynthetically, disrupted and two particulate fractions separated by sucrose-density-gradient centrifugation. The upper particulate fraction, enriched in bacteriochlorophyll, was identified as containing the chromatophores; the lower particulate fraction had the characteristics of the cell envelope. The two fractions differed in cytochrome content and cytochrome spectra. Ferrochelatase was found almost exclusively in the chromatophore fraction and was located on the outer face of the chromatophores, i.e. in contact with the cytosol in intact cells. The addition of 59FeCl3 to cells growing in low-iron media resulted in labelling of the protohaem fraction (probably arising from cytochrome b) of the membranes. The specific radioactivity of the haem of the chromatophores rose more rapidly than that of the envelope fraction and then after 2 h declined to approximately the same value, suggesting that haems of the chromatophore may act as precursors of haem of the envelope.
Full text
PDF
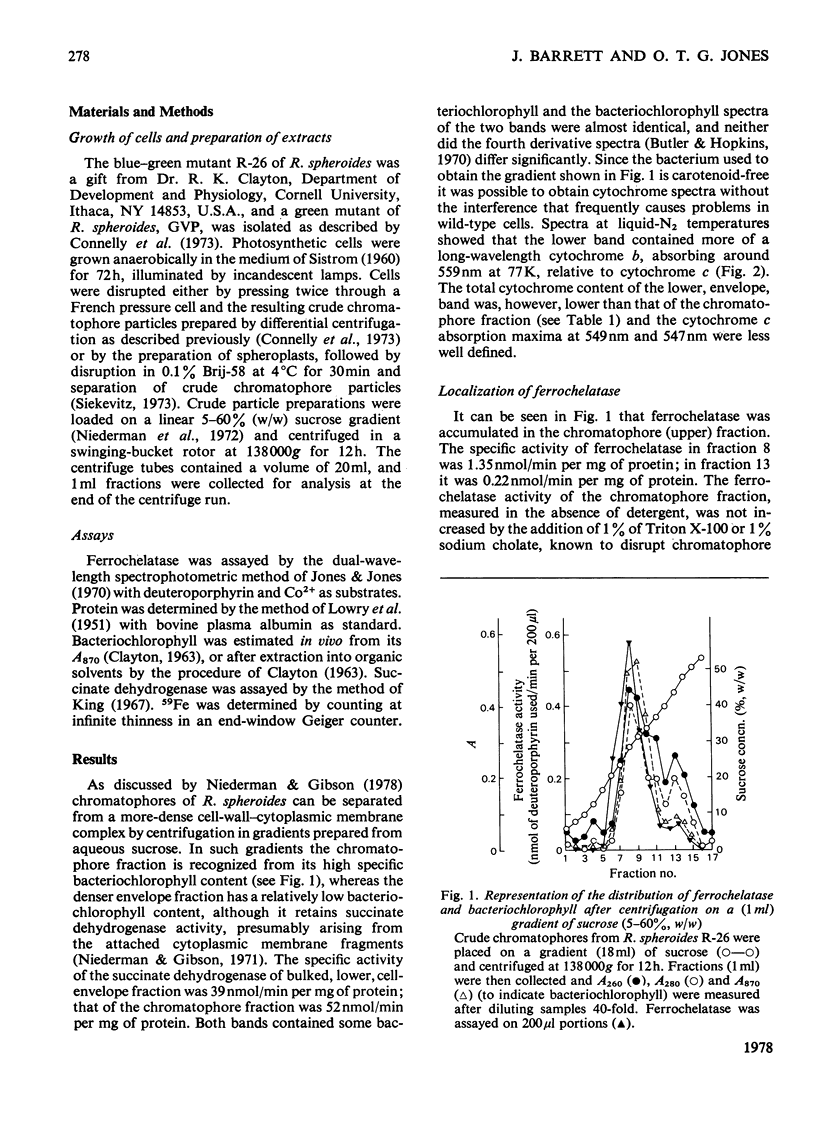
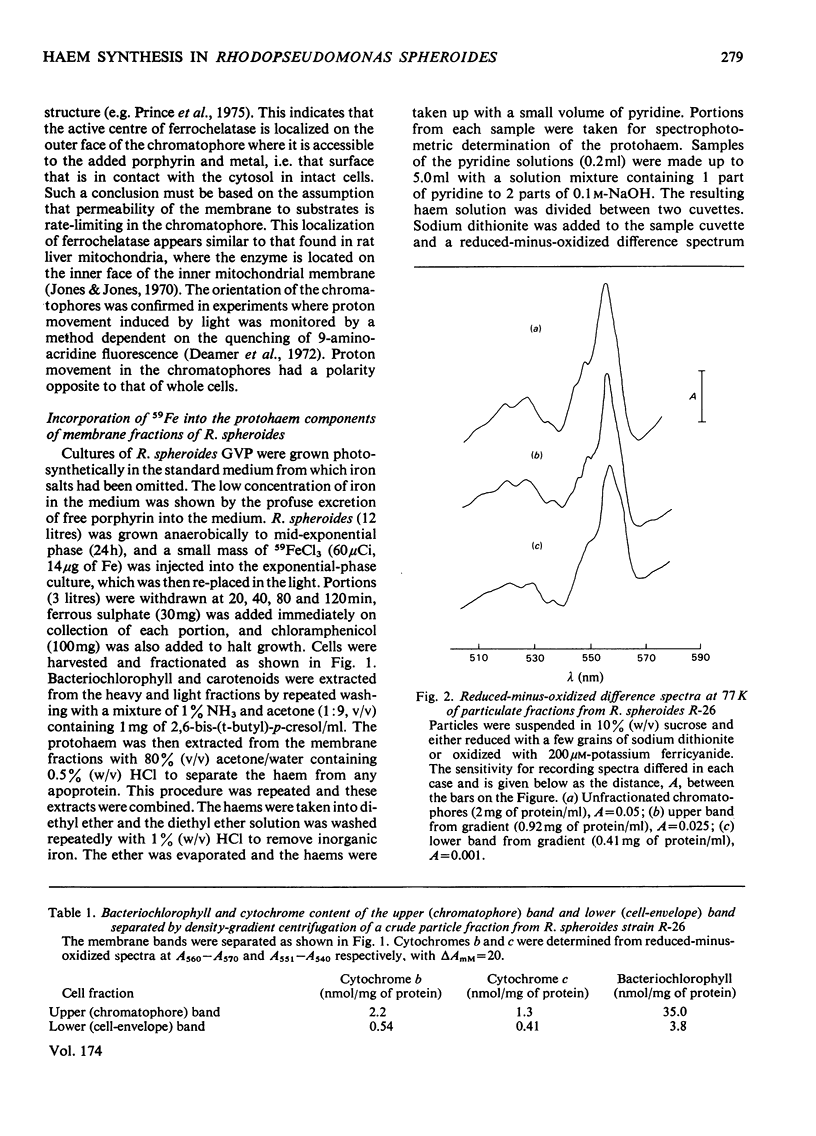
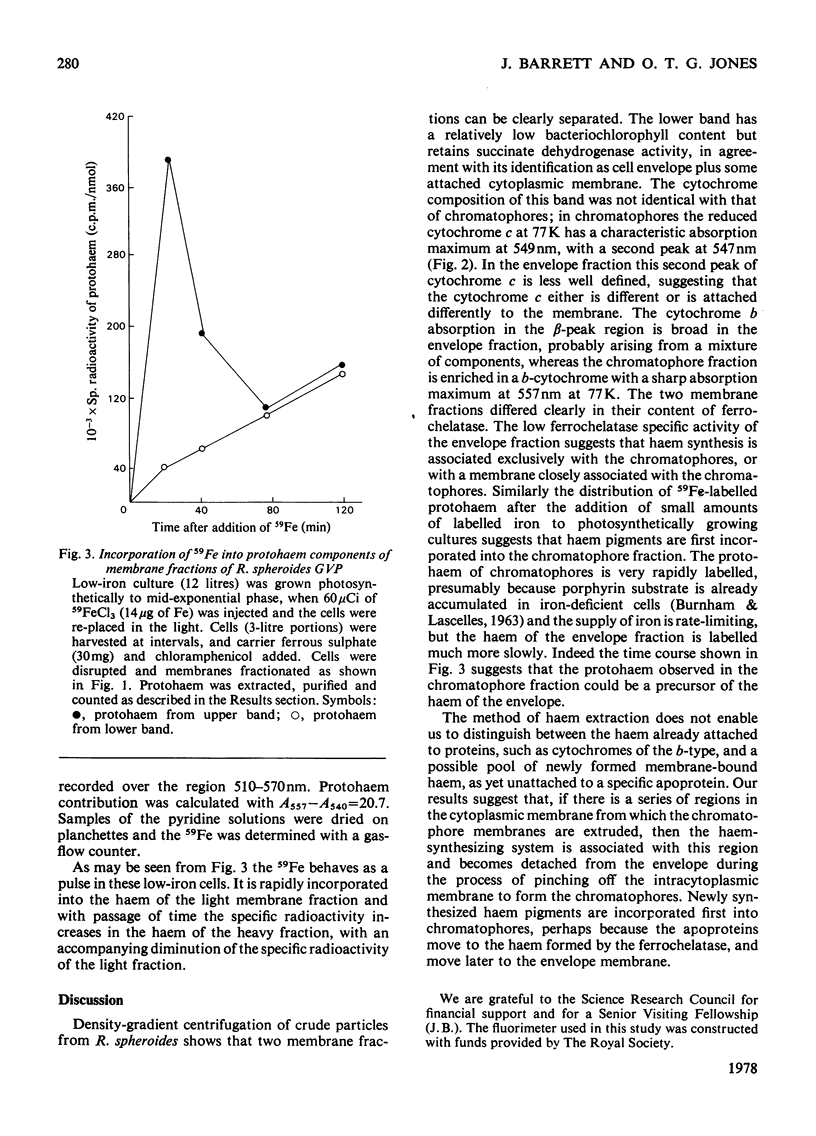

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly J. L., Jones O. T., Saunders V. A., Yates D. W. Kinetic and thermodynamic properties of membrane-bound cytochromes of aerobically and photosynthetically grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Apr 5;292(3):644–653. doi: 10.1016/0005-2728(73)90012-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., Jones O. T. Effect of added light-harvesting pigments on the reconstruction of photosynthetic reactions in membranes from bacteriochlorophyll-less mutants of Rhodopseudomonas sphaeroides. Biochem Soc Trans. 1976;4(4):669–670. doi: 10.1042/bst0040669. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T., Plewis K. M. Reconstitution of light-dependent electron transport in membranes from a bacteriochlorophyll-less mutant of Rhodopseudomonas spheroides. Biochim Biophys Acta. 1974 Aug 23;357(2):204–214. doi: 10.1016/0005-2728(74)90061-9. [DOI] [PubMed] [Google Scholar]
- Kosakowski M. H., Kaplan S. Topology and growth of the intracytoplasmic membrane system of Rhodopseudomonas spheroides: protein, chlorophyll, and phospholipid insertion into steady-state anaerobic cells. J Bacteriol. 1974 Jun;118(3):1144–1157. doi: 10.1128/jb.118.3.1144-1157.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Niederman R. A., Gibson K. D. The separation of chromatophores from the cell envelope in Rhodopseudomonas spheroides. Prep Biochem. 1971;1(2):141–150. doi: 10.1080/00327487108081935. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Segen B. J., Gibson K. D. Membranes of Rhodopseudomonas spheroides. I. Isolation and characterization of membrane fractions from extracts of aerobically and anaerobically grown cells. Arch Biochem Biophys. 1972 Oct;152(2):547–560. doi: 10.1016/0003-9861(72)90250-0. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- VATTER A. E., WOLFE R. S. The structure of photosynthetic bacteria. J Bacteriol. 1958 Apr;75(4):480–488. doi: 10.1128/jb.75.4.480-488.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


