Abstract
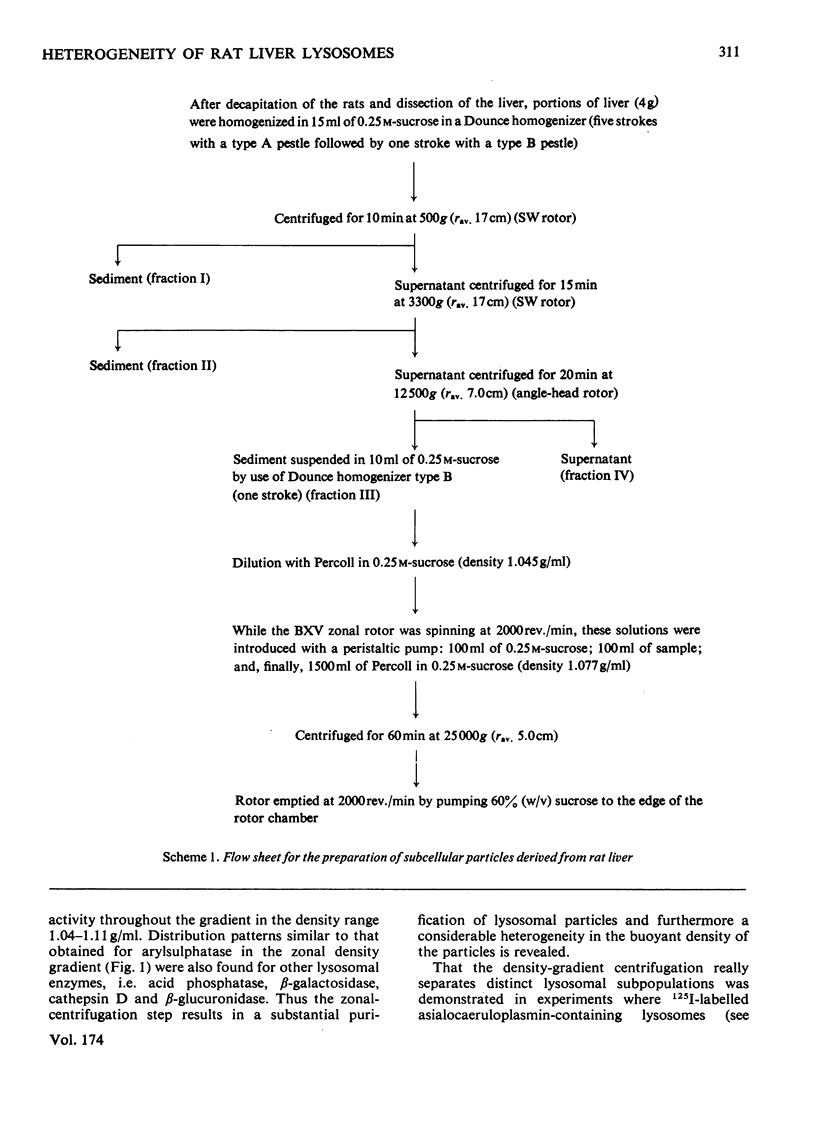
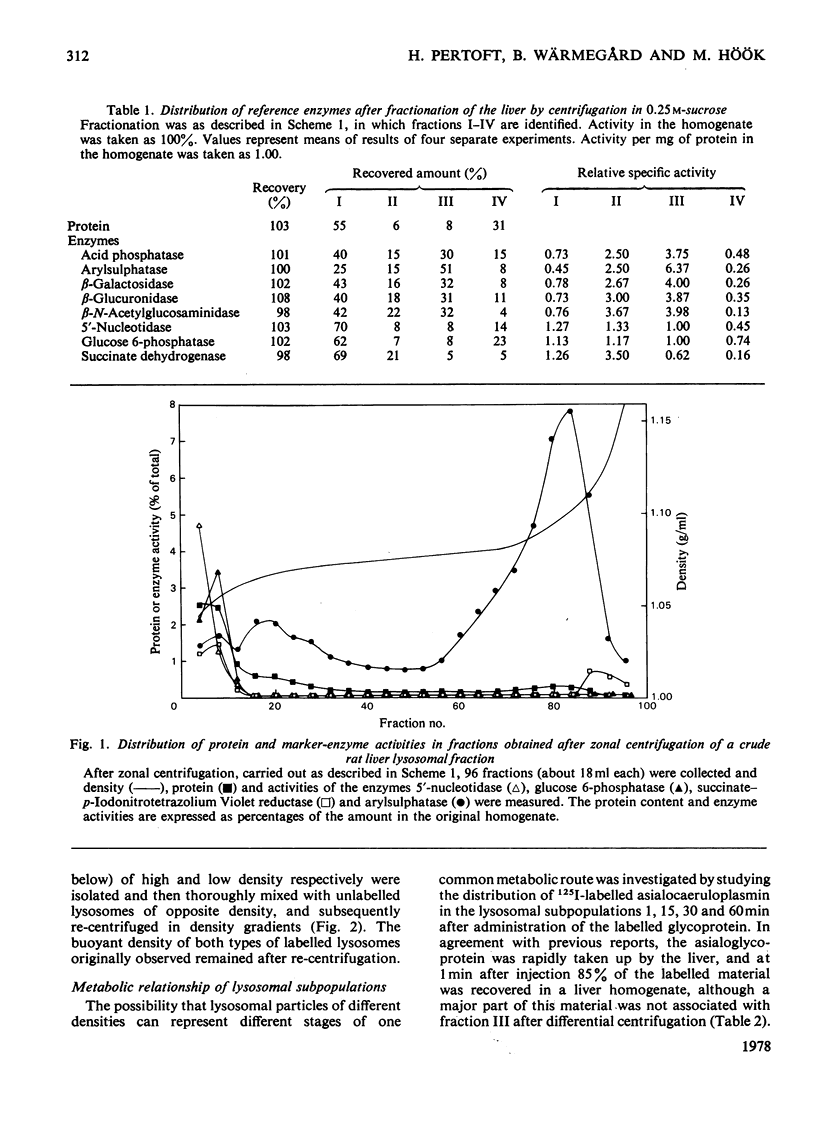
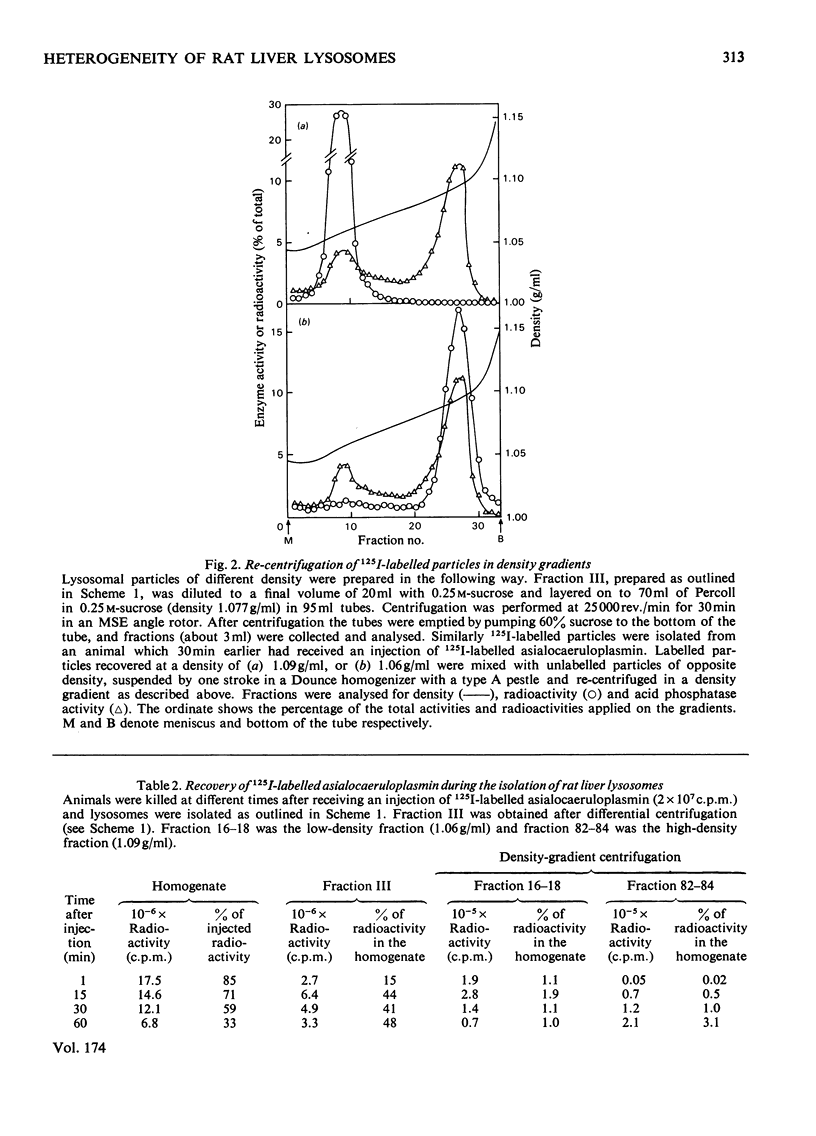
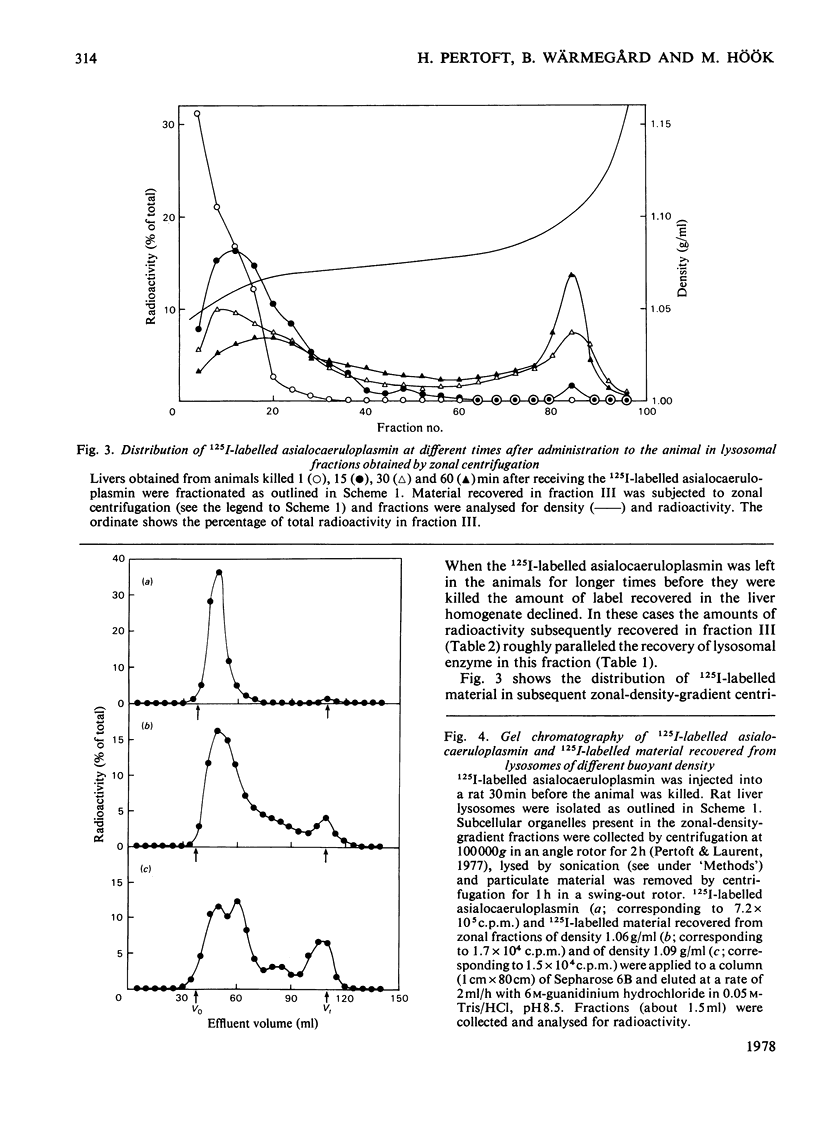
1. A crude lysosomal fraction obtained by differential centrifugation of a rat liver homogenate was subjected to zonal centrifugation in iso-osmotic self-generating gradients composed of modified colloidal silica (Percoll). Analysis of relevant marker-enzyme activities shows a continuous band of considerably purified lysosomal particles in the density range 1.04--1.11 g/ml. 2. A relationship between age and buoyant density of the parenchymal lysosomal subpopulations is indicated by the distribution of 125I-labelled asialoglycoproteins in the heterogeneous lysosomes during the catabolism of the glycoprotein. The labelled asialoglycoprotein first appeared in lysosomal particles of low density, which with time progressively acquired a higher density. Furthermore, 30 min after administration the 125I-labelled asialocaeruloplasmin recovered in the light lysosomes was less degraded than the material recovered in the heavy lysosomes. 3. A lysosomal enzyme (arylsulphatase) was found to possess considerably higher isoelectric points in the heavy lysosomes than in the light lysosomes, which is consistent with a relationship between age and density of the lysosomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborgh B., Berg T., Ericsson J. L. Quantitation of acid phosphatase and aryl sulphatase in rat hepatic parenchymal and kupffer cells. FEBS Lett. 1973 Sep 1;35(1):51–53. doi: 10.1016/0014-5793(73)80574-5. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Baccino F. M., Rita G. A., Zuretti M. F. Studies on the structure-bound sedimentabolity of some rat liver lysosome hydrolases. Biochem J. 1971 Apr;122(3):363–371. doi: 10.1042/bj1220363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Boman D. Distribution of lysosomal enzymes between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1973 Oct 10;321(2):585–596. doi: 10.1016/0005-2744(73)90201-5. [DOI] [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond J. D., Johnston R. G., Kidd D., Rylance H. J., Sommerville R. G. The inhibition of neuraminidase and antiviral action. Br J Pharmacol Chemother. 1966 Aug;27(2):415–426. doi: 10.1111/j.1476-5381.1966.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Tsung P. K., Mizuno D. Possible heterogeneity of the distribution of lysosomal marker enzymes among "lysosomal particles" of rat liver. Biochim Biophys Acta. 1971 Feb 28;261(2):508–516. doi: 10.1016/0304-4165(72)90075-x. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Synthesis and turnover of lysosomal glycoproteins. Relation to the molecular heterogeneity of the lysosomal enzymes. FEBS Lett. 1974 Feb 15;39(2):176–181. doi: 10.1016/0014-5793(74)80045-1. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Morell A. G., Sternlieb I., Scheinberg I. H. Catabolism of desialylated ceruloplasmin in the liver. J Biol Chem. 1970 Nov 10;245(21):5833–5837. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl P., Hjertén S. Isoelectric focusing in free Ampholine solution and attempts at isoelectric focusing in pH gradients created in ordinary buffers. Ann N Y Acad Sci. 1973 Jun 15;209:94–111. doi: 10.1111/j.1749-6632.1973.tb47521.x. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Van den Hamer C. J., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal D-galactose residues. J Biol Chem. 1966 Aug 25;241(16):3745–3749. [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. The permeability properties of the lysosomal membrane. Biochim Biophys Acta. 1977 Nov 14;472(3-4):419–449. doi: 10.1016/0304-4157(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Robins E., Hirsch H. E., Emmons S. S. Glycosidases in the nervous system. I. Assay, some properties, and distribution of beta-galactosidase, beta-glucoronidase, and beta-glucosidase. J Biol Chem. 1968 Aug 25;243(16):4246–4252. [PubMed] [Google Scholar]
- Sherman W. R., Stanfield E. F. Measurement of the arylsulphatase of Patella vulgata with 4-methylumbelliferone sulphate. Biochem J. 1967 Mar;102(3):905–909. doi: 10.1042/bj1020905. [DOI] [PMC free article] [PubMed] [Google Scholar]


