Abstract
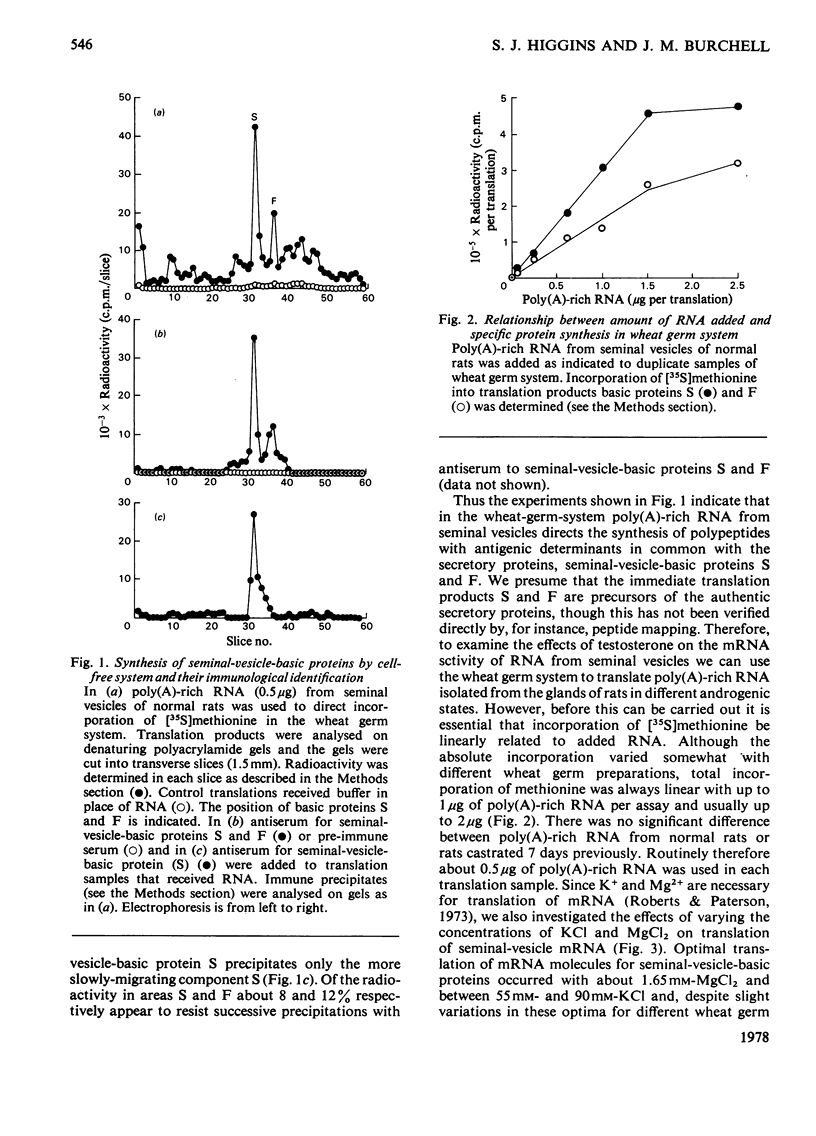
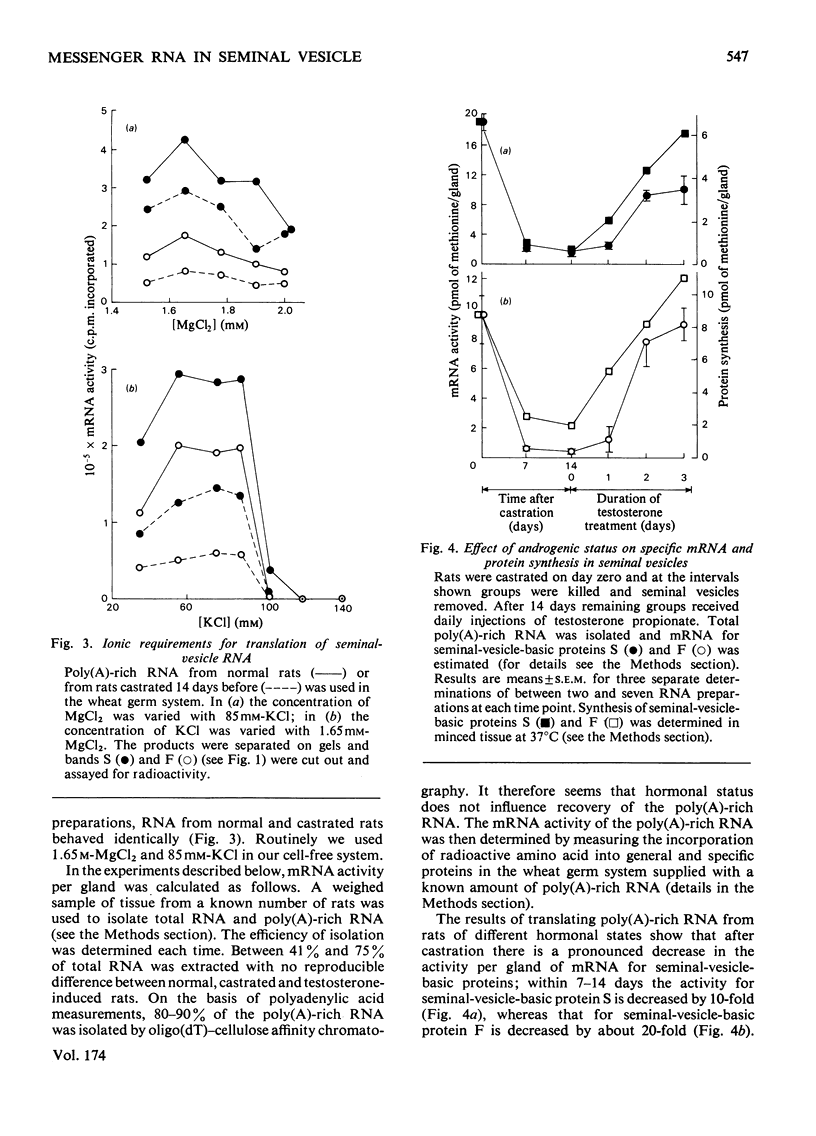
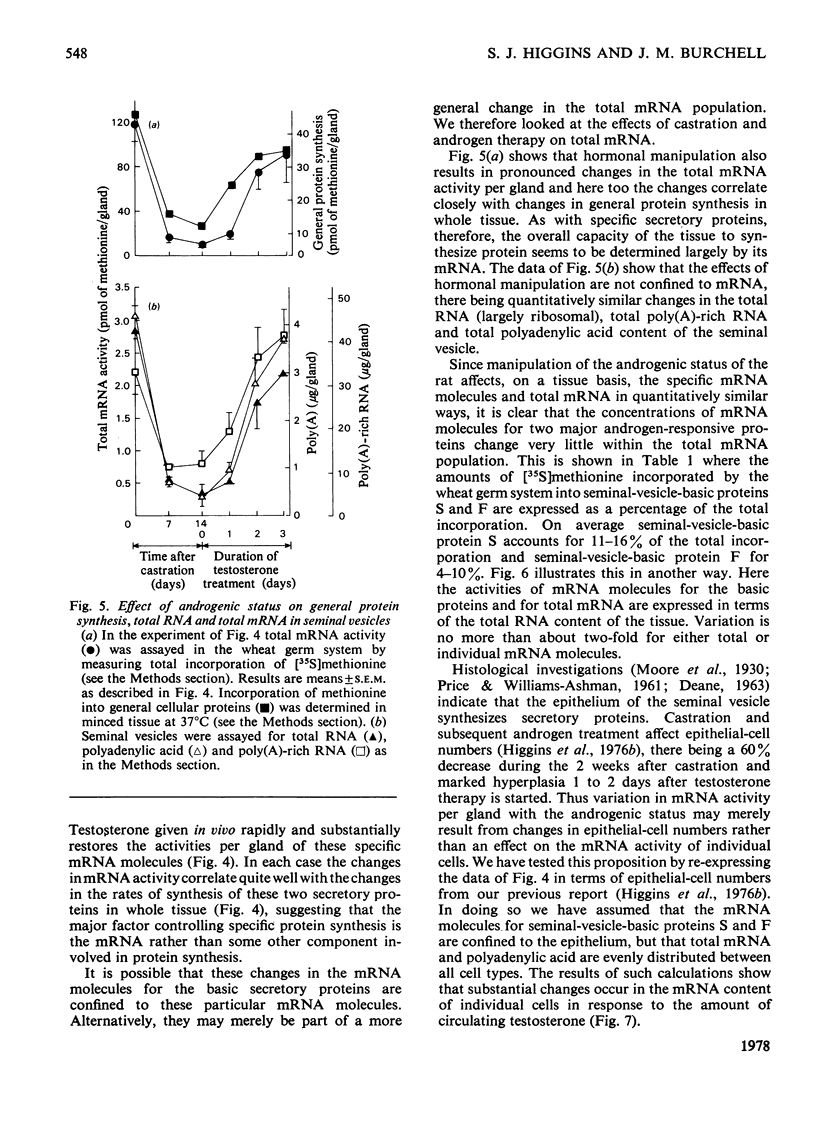
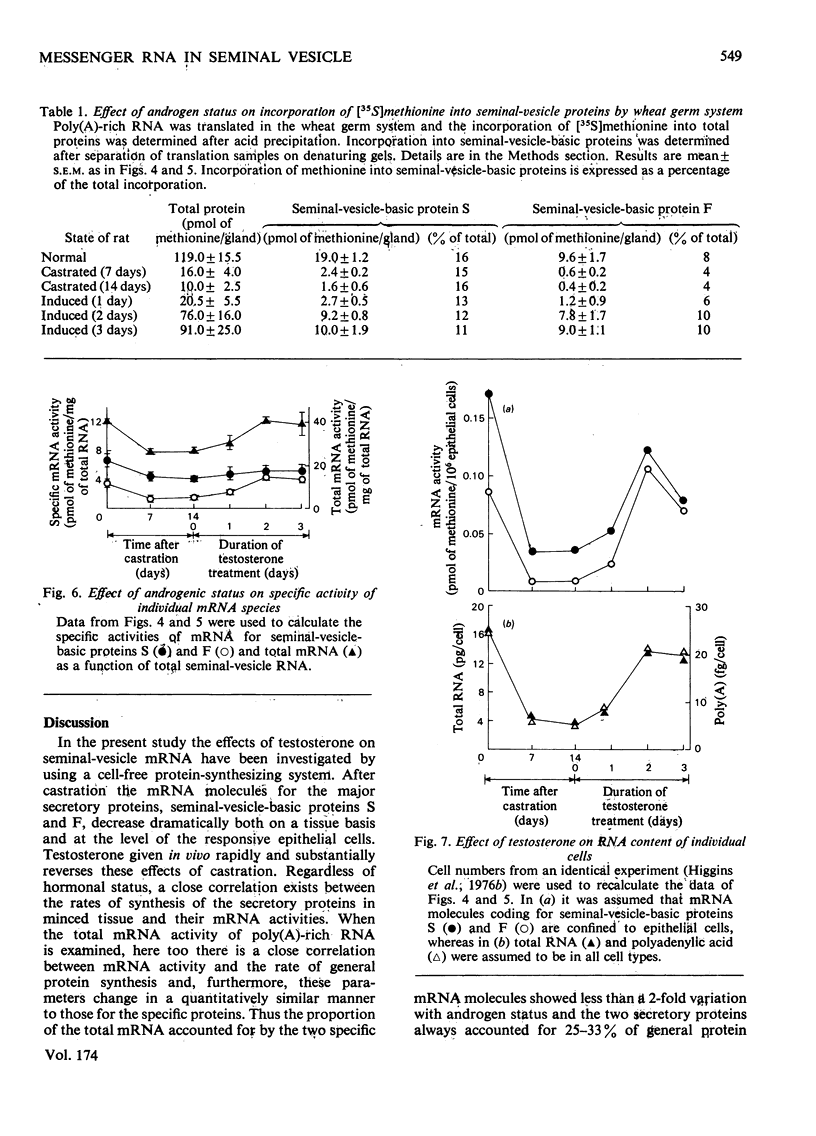
In a previous report [Higgins et al. (1976) Biochem. J. 158, 271–282] we described the effects of alterations in androgen status on the synthesis of two basic secretory proteins of the rat seminal vesicle. In the present paper we examine the effects of testosterone on the activity of mRNA in the seminal vesicle. Total cellular poly(A)-rich RNA was isolated and translated in a cell-free system prepared from wheat germ. Translation products were separated on denaturing polyacrylamide gels and the protein bands corresponding to the two basic secretory proteins were identified immunologically. Incorporation of radioactive methionine into these bands was taken as a measure of the individual mRNA activities. Total mRNA activity was estimated by radioactivity in total acid-precipitable material. The results show that 1 to 2 weeks after castration the activities of mRNA molecules for the basic secretory proteins were decreased 10–20-fold on a tissue basis. Testosterone given in vivo rapidly and substantially restores mRNA activity to normal. Since these changes correlate closely with variations in the rates of synthesis of the secretory proteins in whole cells it suggests that androgenic steroids control protein synthesis chiefly via mRNA availability. In this respect their action resembles those of other steroid hormones acting in other systems. However, these effects of testosterone on the mRNA molecules for the major secretory proteins could not be distinguished from those on total mRNA. Thus the proportion of the total mRNA population accounted for by the two specific mRNA molecules showed less than a 2-fold variation with androgen status. Similarly the two secretory proteins always accounted for 25–33% of general protein synthesis. This is in sharp contrast with the markedly differential effects of other steroid hormones controlling synthesis of major proteins in other well-studied systems. We interpret our results as indicating that testosterone regulates the mRNA population of the seminal vesicle as a whole.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bancroft F. C., Tashjian A. H., Jr Growth in suspension culture of rat pituitary cells which produce growth hormone and prolactin. Exp Cell Res. 1971 Jan;64(1):125–128. doi: 10.1016/0014-4827(71)90201-1. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- DEANE H. W. ELECTRON MICROSCOPIC OBSERVATIONS ON THE MOUSE SEMINAL VESICLE. Natl Cancer Inst Monogr. 1963 Oct;12:63–83. [PubMed] [Google Scholar]
- Dannies P. S., Tashjian A. R., Jr Effects of thyrotropin-releasing hormone and hydrocortisone on synthesis and degradation of prolactin in a rat pituitary cell strain. J Biol Chem. 1973 Sep 10;248(17):6174–6179. [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Williams J. G., Penman S. Message and non-message sequences adjacent to poly(A) in steady state heterogeneous nuclear RNA of HeLa cells. Cell. 1976 Mar;7(3):429–437. doi: 10.1016/0092-8674(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. Biochem J. 1976 Aug 15;158(2):271–282. doi: 10.1042/bj1580271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Baglioni C. Messenger RNA for globin in the postribosomal supernatant of rabbit reticulocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1425–1428. doi: 10.1073/pnas.69.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kohler P. O., Frohman L. A., Bridson W. E., Vanha-Perttula T., Hammond J. M. Cortisol induction of growth hormone synthesis in a clonal line of rat pituitary tumor cells in culture. Science. 1969 Oct 31;166(3905):633–634. doi: 10.1126/science.166.3905.633. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Ansah-Yiadom R., Feigelson P. Effects of sex hormones on the level of the messenger RNA for the rat hepatic protein alpha 2u globulin. J Biol Chem. 1976 Jun 25;251(12):3594–3598. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Effect of estrogen on the sequence and population complexity of chick oviduct poly(A)-containing RNA. J Biol Chem. 1976 Jun 25;251(12):3738–3748. [PubMed] [Google Scholar]
- Mullinix K. P., Wetekam W., Deeley R. G., Gordon J. I., Meyers M., Kent K. A., Goldberger R. F. Induction of vitellogenin synthesis by estrogen in avian liver: relationship between level of vitellogenin mRNA and vitellogenin synthesis. Proc Natl Acad Sci U S A. 1976 May;73(5):1442–1446. doi: 10.1073/pnas.73.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Rosenfeld G. C., Comstock J. P., Means A. R. Steroid hormone induction of a specific translatable messenger RNA. Nat New Biol. 1972 Nov 8;240(97):45–48. doi: 10.1038/newbio240045a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Parker M. G., Mainwaring W. I. Effects of androgens on the complexity of poly(A) RNA from rat prostate. Cell. 1977 Oct;12(2):401–407. doi: 10.1016/0092-8674(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T. The androgenic regulation of abundant mRNA in rat ventral prostate. Eur J Biochem. 1978 Apr 17;85(2):399–406. doi: 10.1111/j.1432-1033.1978.tb12252.x. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Killewich L., Chen G., Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1017–1020. doi: 10.1073/pnas.72.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. Kinetics of steroid induction and deinduction of tyrosine aminotransferase synthesis in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2007–2011. doi: 10.1073/pnas.72.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Thompson E. B., Hayashi S., Gelehrter T., Granner D., Peterkofsky B. Tyrosine transaminase induction in mammalian cells in tissue culture. Cold Spring Harb Symp Quant Biol. 1966;31:349–360. doi: 10.1101/sqb.1966.031.01.045. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Sussman P. M., Yu L. Y., Bancroft F. C. Pregrowth hormone messenger RNA: glucocorticoid induction and identification in rat pituitary cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2357–2361. doi: 10.1073/pnas.74.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T., Bayley S. T., Harvey R., Crawford L. V., Smith A. E. Cell-free synthesis of polyoma virus capsid proteins VP1 and VP2. J Virol. 1977 Jan;21(1):215–224. doi: 10.1128/jvi.21.1.215-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


