Abstract
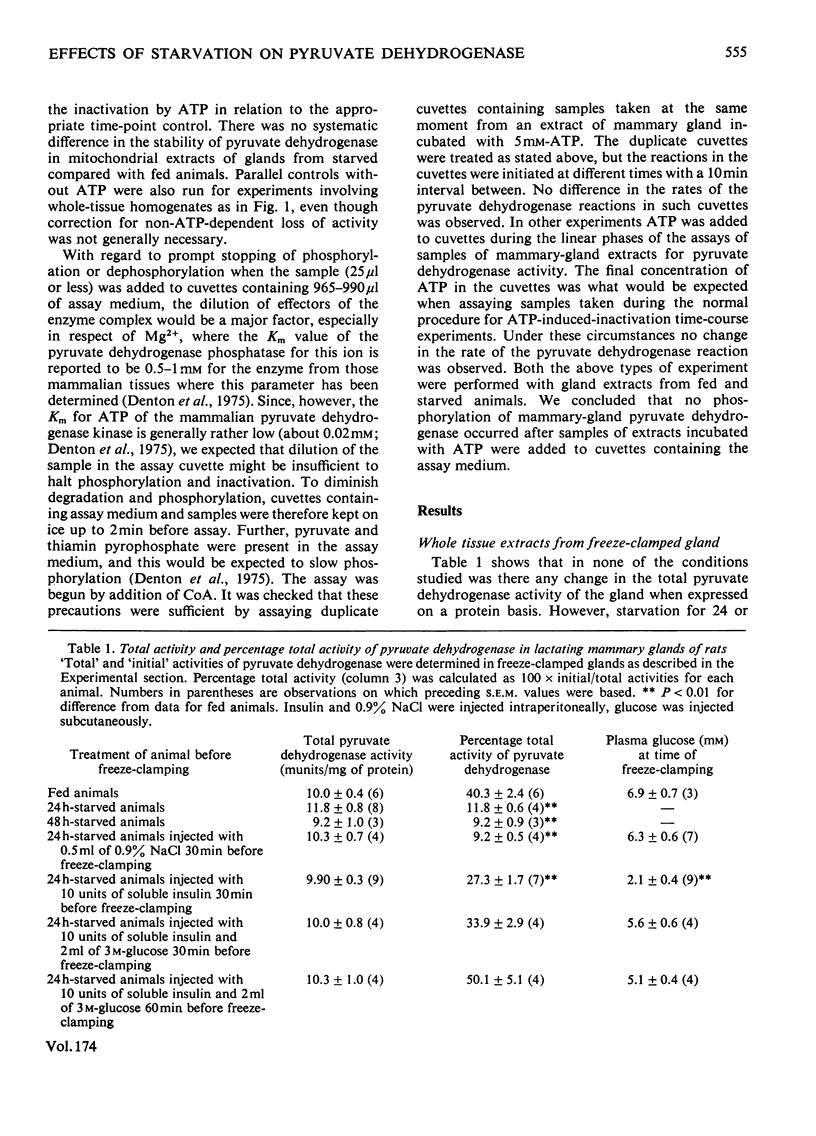
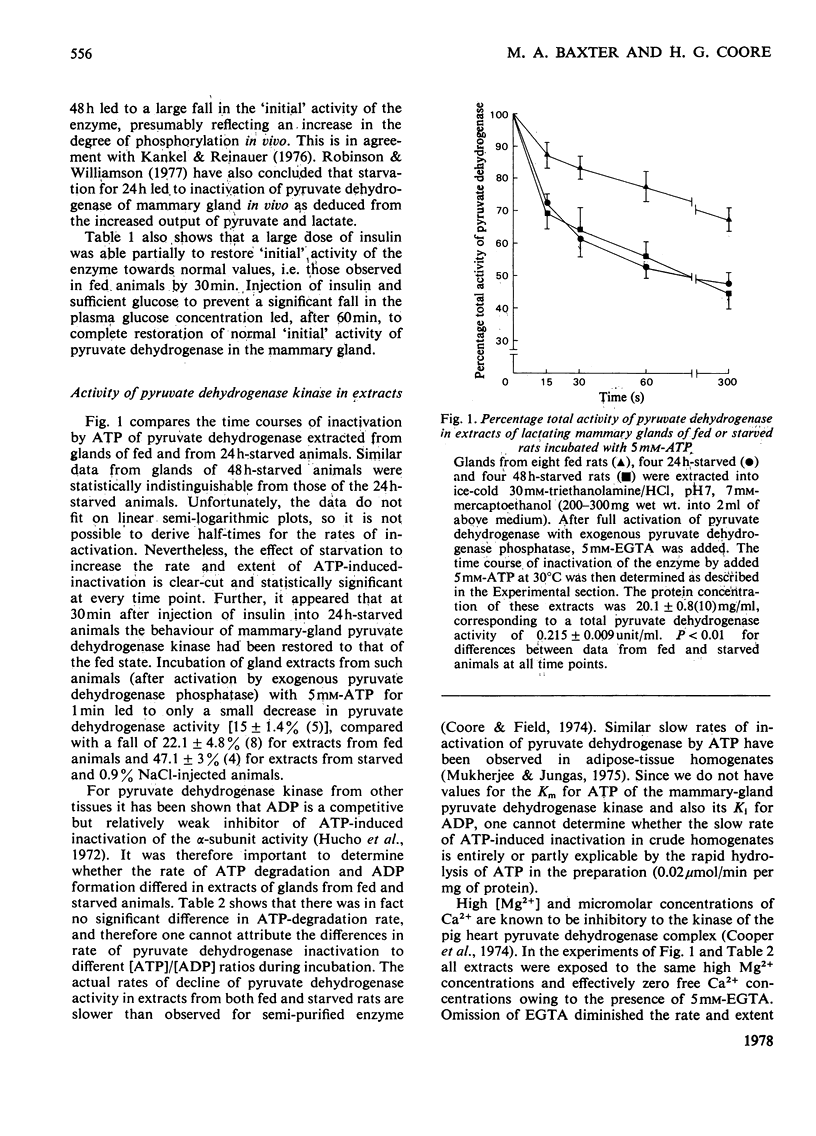
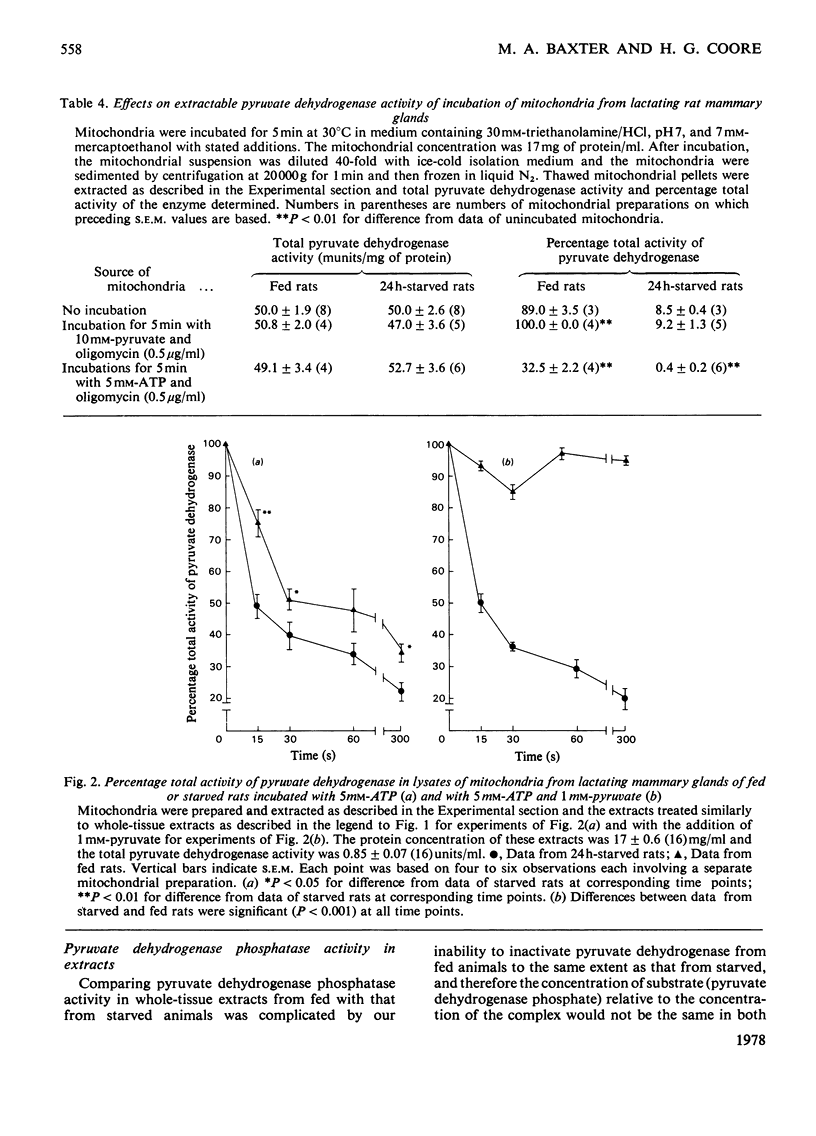
1. The `initial activity' of the pyruvate dehydrogenase enzyme complex in whole tissue or mitochondrial extracts of lactating rat mammary glands was greatly decreased by 24 or 48h starvation of the rats. Injection of insulin and glucose into starved rats 60min before removal of the glands abolished this difference in `initial activities'. 2. The `total activity' of the enzyme complex in such extracts was revealed by incubation in the presence of free Mg2+ and Ca2+ ions (more than 10 and 0.1mm respectively) and a crude preparation of pig heart pyruvate dehydrogenase phosphatase. Starvation did not alter this `total activity'. It is assumed that the decline in `initial activity' of the enzyme complex derived from the glands of starved animals was due to increased phosphorylation of its α-subunit by intrinsic pyruvate dehydrogenase kinase. 3. Starvation led to an increase in intrinsic pyruvate dehydrogenase kinase activity in both whole tissue and mitochondrial extracts. Injection of insulin into starved animals 30min before removal of the lactating mammary glands abolished the increase in pyruvate dehydrogenase kinase activity in whole-tissue extracts. 4. Pyruvate (1mm) prevented ATP-induced inactivation of the enzyme complex in mitochondrial extracts from glands of fed animals. In similar extracts from starved animals pyruvate was ineffective. 5. Starvation led to a decline in activity of pyruvate dehydrogenase phosphatase in mitochondrial extracts, but not in whole-tissue extracts. 6. These changes in activity of the intrinsic kinase and phosphatase of the pyruvate dehydrogenase complex of lactating rat mammary gland are not explicable by current theories of regulation of the complex.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter M. A., Coore H. G. Persistent effects of starvation on pyruvate dehydrogenase kinase and phosphatase of lactating rat mammary gland [proceedings]. Biochem Soc Trans. 1978;6(1):154–157. doi: 10.1042/bst0060154. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Field B. Properties of pyruvate dehydrogenase of rat mammary tissue and its changes during pregnancy, lactation and weaning. Biochem J. 1974 Jul;142(1):87–95. doi: 10.1042/bj1420087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Field B., Coore H. G. Control of rat mammary-gland pyruvate dehydrogenase by insulin and prolactin. Biochem J. 1976 May 15;156(2):333–337. doi: 10.1042/bj1560333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Wieland O. H. Metabolism of isolated kidney tubules. Regulation of pyruvate dehydrogenase by metabolic substrates. Eur J Biochem. 1974 Mar 1;42(2):529–538. doi: 10.1111/j.1432-1033.1974.tb03368.x. [DOI] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Effect of insulin on fatty acid ynthesis from pyruvate, lactage, or endogenous sources in adipose tissue: evidence for the hormonal regulation of pyruvate dehydrogenase. Endocrinology. 1970 Jun;86(6):1368–1375. doi: 10.1210/endo-86-6-1368. [DOI] [PubMed] [Google Scholar]
- Kankel K. F., Reinauer H. Activity of pyruvate dehydrogenase complex in the mammary gland of normal and diabetic rats. Diabetologia. 1976 May;12(2):149–154. doi: 10.1007/BF00428981. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J. Diabetes and the control of pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidized nicotinamide-adenine dinucleotide and of acetyl-coenzyme A/coenzyme A. Biochem J. 1977 Jun 15;164(3):509–519. doi: 10.1042/bj1640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Jungas R. L. Activation of pyruvate dehydrogenase in adipose tissue by insulin. Evidence for an effect of insulin on pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1975 May;148(2):229–235. doi: 10.1042/bj1480229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Löffler G., Wieland O. H. Interconversion of pyruvate dehydrogenase in the isolated perfused rat liver. Eur J Biochem. 1973 Feb 15;33(1):117–122. doi: 10.1111/j.1432-1033.1973.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Portenhauser R., Wieland O. Regulation of pyruvate dehydrogenase in mitochondria of rat liver. Eur J Biochem. 1972 Dec 4;31(2):308–314. doi: 10.1111/j.1432-1033.1972.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Goheer M. A., Coore H. G., Mayes P. A. Regulation by insulin and free fatty acids of pyruvate dehydrogenase activity in perfused rat liver [proceedings]. Biochem Soc Trans. 1977;5(4):1000–1001. doi: 10.1042/bst0051000. [DOI] [PubMed] [Google Scholar]
- Wieland O., Funcke H. v., Löffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett. 1971 Jul 1;15(4):295–298. doi: 10.1016/0014-5793(71)80641-5. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]


