Abstract
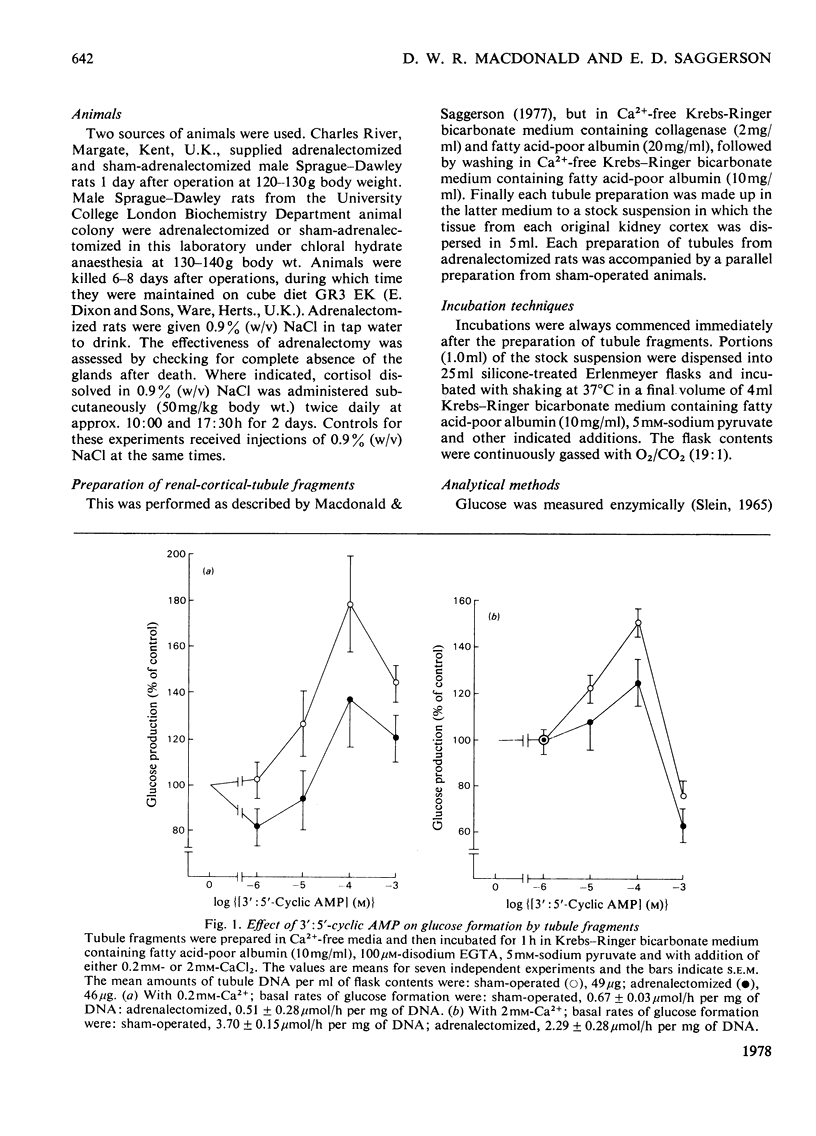
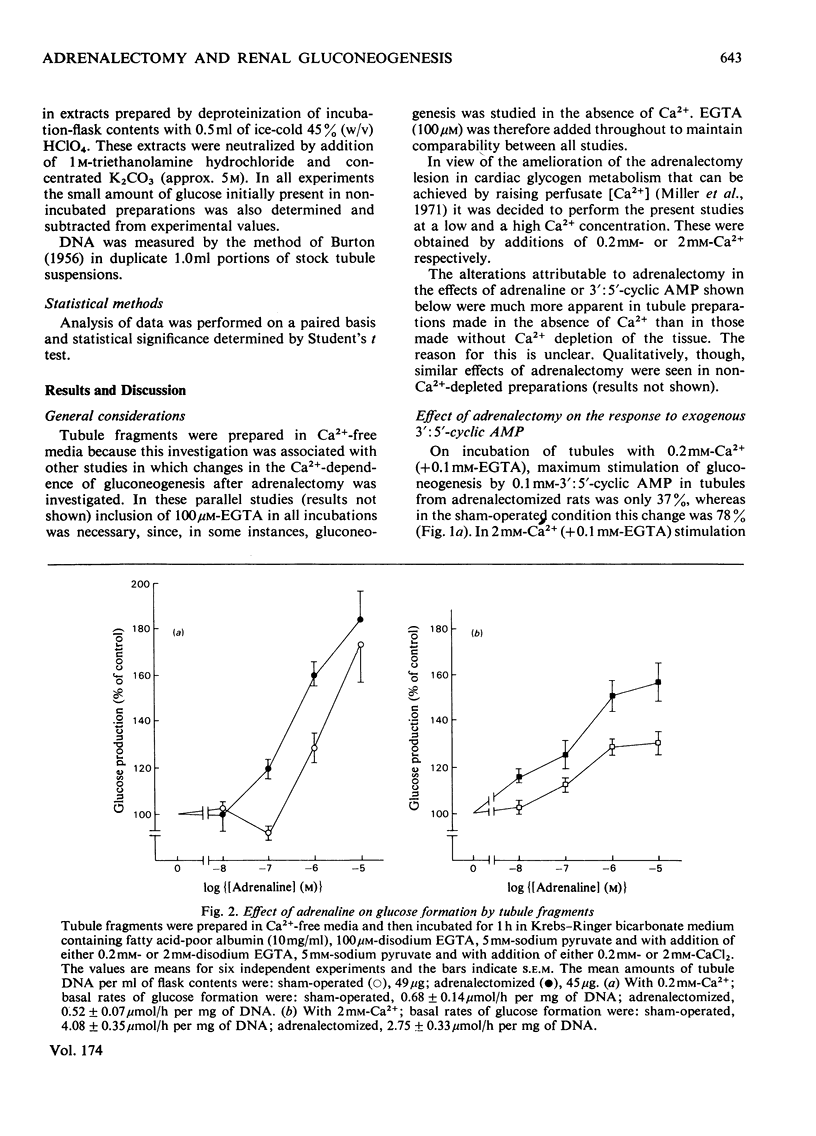
1. Gluconeogenesis from pyruvate was measured in renal-cortical-tubules fragments prepared from fed male rats 6-8 days after adrenalectomy or sham adrenalectomy. The response of this process to 3':5'-cyclic AMP and adrenaline was compared in these two states at two Ca2+ concentrations. 2. Adrenalectomy decreased the percentage stimulation of gluconeogenesis by 3':5'-cyclic AMP, but increased percentage stimulation by adrenaline. Cortisol treatment of adrenalectomized rats (50 mg/kg, twice daily for 2 days) did not reverse the change in responsiveness to 3':5'-cyclic AMP and adrenaline. 3. Stimulation of gluconeogenesis by 1 micron-adrenaline was unaffected by 10 micron-propranolol (beta-blocker) in either state. Phentolamine (alpha-blocker; 10 micron) totally blocked stimulation of gluconeogenesis by 1 micron-adrenaline in the sham-operated condition, but was only partially effective in this respect after adrenalectomy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. O., Beck R. R. Alterations in lipolysis, adenylate cyclase and adenosine 3', 5'-monophosphate levels in isolated fat cells following adrenalectomy. Endocrinology. 1972 Aug;91(2):504–510. doi: 10.1210/endo-91-2-504. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A., Flores H., Roobol A. The interrelationship of the concentration of hydrogen ions, bicarbonate ions, carbon dioxide and calcium ions in the regulation of renal gluconeogenesis in the rat. Biochem J. 1973 Nov;136(3):445–453. doi: 10.1042/bj1360445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Exton J. H., Friedmann N., Wong E. H., Brineaux J. P., Corbin J. D., Park C. R. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J Biol Chem. 1972 Jun 10;247(11):3579–3588. [PubMed] [Google Scholar]
- Exton J. H., Harper S. C. Role of cyclic AMP in the actions of catecholamines on hepatic carbohydrate metabolism. Adv Cyclic Nucleotide Res. 1975;5:519–532. [PubMed] [Google Scholar]
- Guder W. G., Rupprecht A. Metabolism of isolated kidney tubules. Independent actions of catecholamines on renal cyclic adenosine 3':5'-monophosphate levels and gluconeogenesis. Eur J Biochem. 1975 Mar 17;52(2):283–290. doi: 10.1111/j.1432-1033.1975.tb03996.x. [DOI] [PubMed] [Google Scholar]
- Guder W., Wiesner W., Stukowski B., Wieland O. Metabolism of isolated kidney tubules. Oxygen consumption, gluconeogenesis and the effect of cyclic nucleotides in tubules from starved rats. Hoppe Seylers Z Physiol Chem. 1971 Oct;352(10):1319–1328. doi: 10.1515/bchm2.1971.352.2.1319. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D. W., Saggerson E. D. Hormonal control of gluconeogenesis in tubule fragments from renal cortex of fed rats. Effects of alpha-adrenergic stimuli, glucagon, theophylline and papaverine. Biochem J. 1977 Oct 15;168(1):33–42. doi: 10.1042/bj1680033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. B., Exton J. H., Park C. R. A block in epinephrine-induced glycogenolysis in hearts from adrenalectomized rats. J Biol Chem. 1971 Jun 10;246(11):3672–3678. [PubMed] [Google Scholar]
- Nagata N., Rasmussen H. Renal gluconeogenesis: effects of Ca2+ and H+. Biochim Biophys Acta. 1970 Jul 21;215(1):1–16. doi: 10.1016/0304-4165(70)90382-x. [DOI] [PubMed] [Google Scholar]
- RUTMAN J. Z., MELTZER L. E., KITCHELL J. R., RUTMAN R. J., GEORGE P. EFFECT OF METAL IONS ON IN VITRO GLUCONEOGENESIS IN RAT KIDNEY CORTEX SLICES. Am J Physiol. 1965 May;208:842–846. doi: 10.1152/ajplegacy.1965.208.5.841. [DOI] [PubMed] [Google Scholar]
- Roobol A., Alleyne G. A. Regulation of renal gluconeogenesis by calcium ions, hormones and adenosine 3':5'-cyclic monophosphate. Biochem J. 1973 May;134(1):157–165. doi: 10.1042/bj1340157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y., Ui M. Activation and inactivation of phosphorylase and glycogen synthetase during perfusion of rat liver as influenced by epinephrine, glucagon and hydrocortisone. Biochim Biophys Acta. 1975 Sep 8;404(1):7–17. doi: 10.1016/0304-4165(75)90142-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer L. D., Chenoweth M., Dunn A. Adrenal corticosteroid involvement in the control of liver glycogen phosphorylase activity. Biochim Biophys Acta. 1969 Nov 18;192(2):292–303. doi: 10.1016/0304-4165(69)90368-7. [DOI] [PubMed] [Google Scholar]


