Abstract
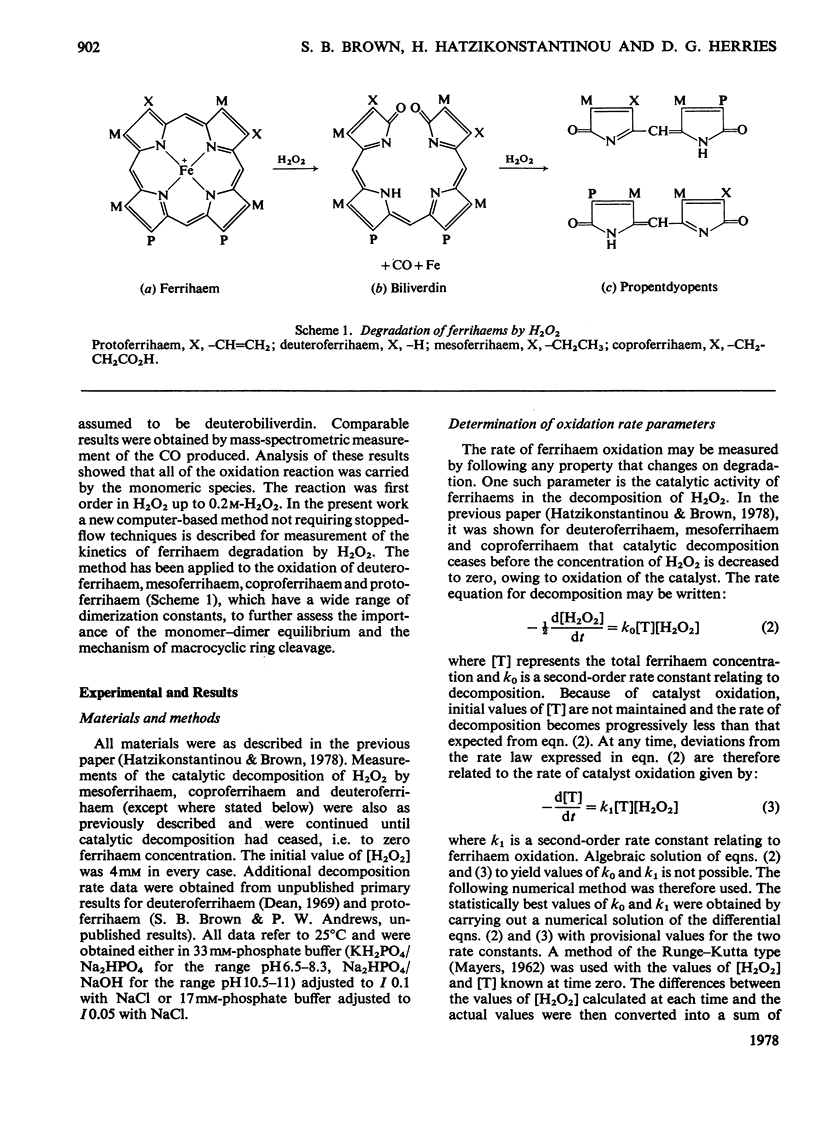
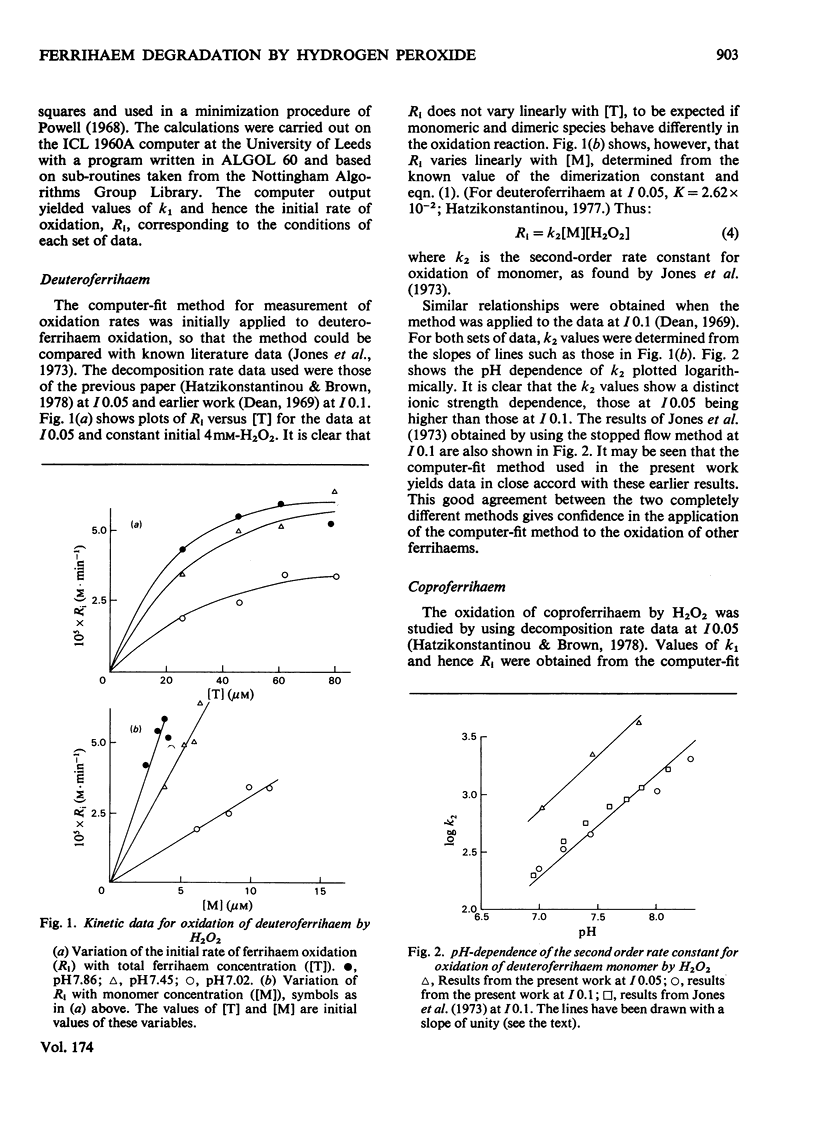
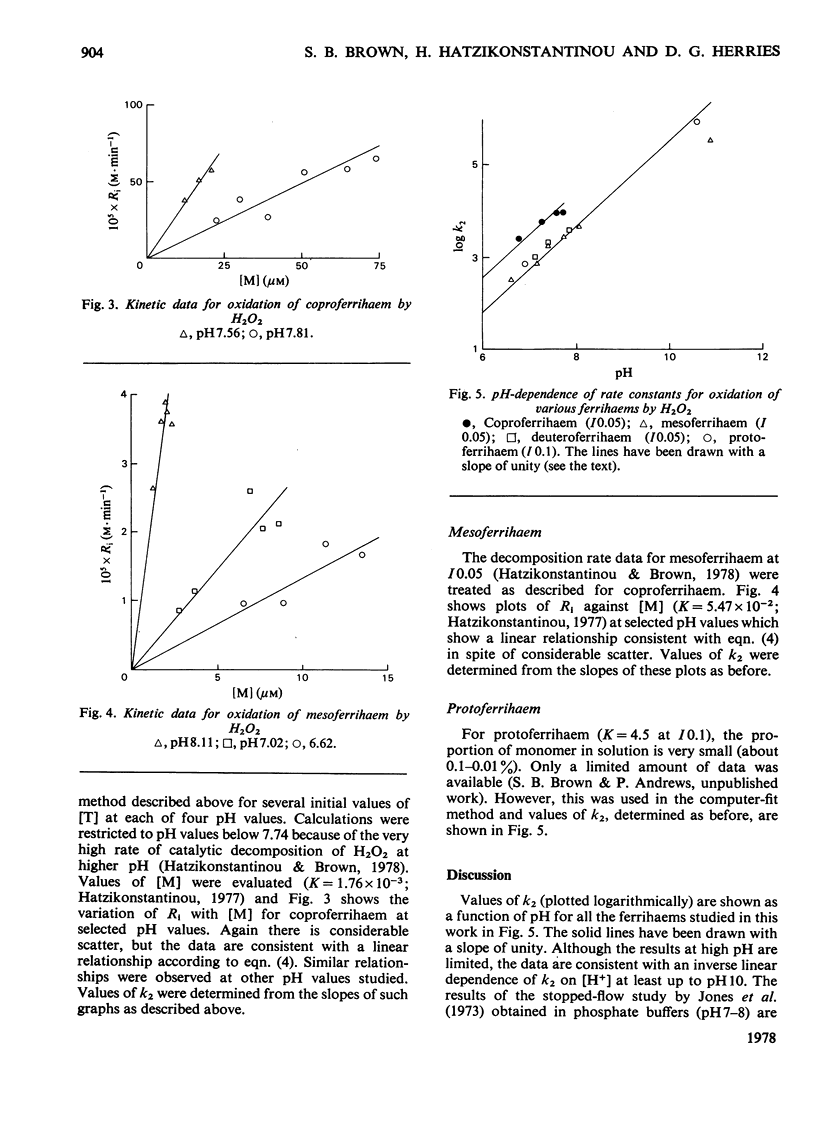
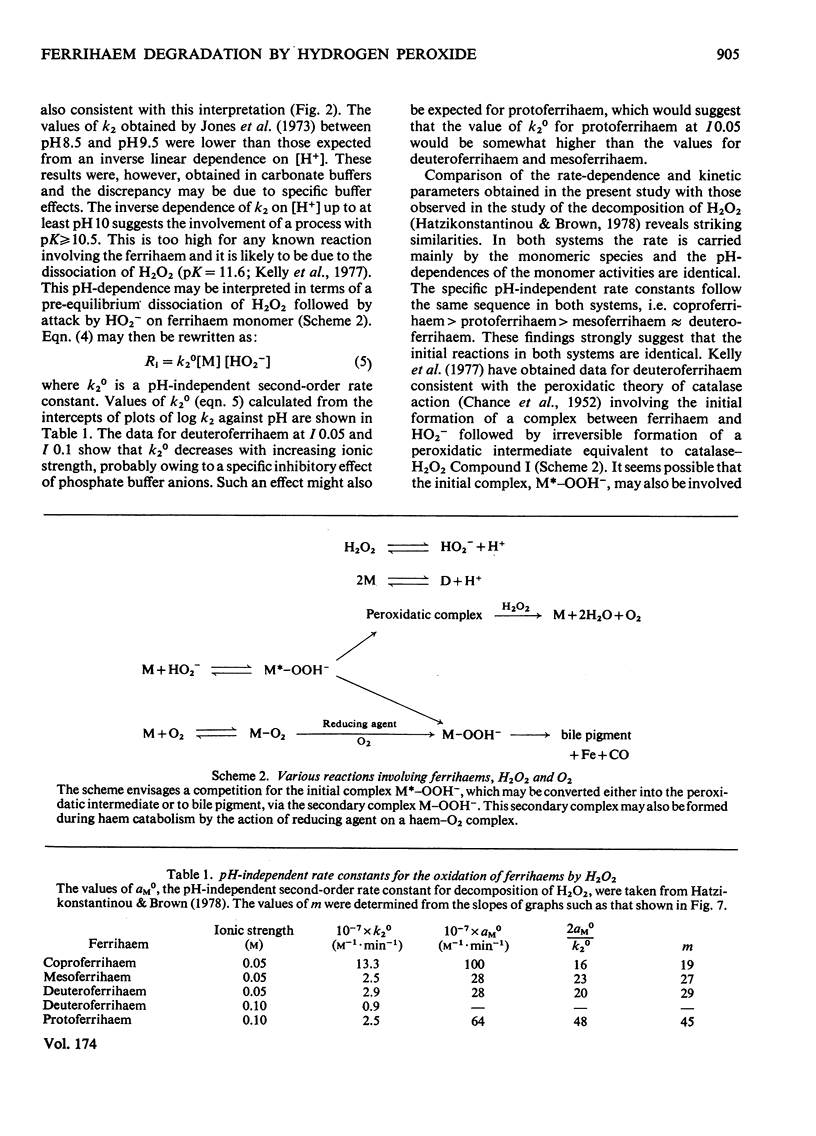
The oxidation of ferrihaems by H2O2 was studied as a model for haem catabolism. Rates of ferrihaem oxidation were evaluated by using a new computer-based method that measures the loss in catalytic activity of the ferrihaem during oxidation. For protoferrihaem, deuteroferrihaem, coproferrihaem and mesoferrihaem, oxidation proceeded via the monomeric species and no dimer contribution was detectable. The pH-dependence of oxidation was studied in the range 6.5--11. Within experimental error, the data were compatible with an inverse linear dependence on [H+]. This was interpreted in terms of attack by HO2- on monomeric ferrihaem. The specific second-order rate constants for oxidation of monomeric species by HO2- were of the same order of magnitude for all the ferrihaems, and were in the sequence coproferrihaem greater than protoferrihaem greater than mesoferrihaem congruent to deuteroferrihaem. A model is suggested involving formation of a ferrihaem monomerperoxide complex, which may either dissociate with the formation of a peroxidatic intermediate or be involved in an intramolecular oxidation of the ferrihaem. Haem catabolism may occur via the same or a similar intermediate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Grundy M. S. The role of iron in haem degradation [proceedings]. Biochem Soc Trans. 1977;5(4):1017–1020. doi: 10.1042/bst0051017. [DOI] [PubMed] [Google Scholar]
- Brown S. B., King R. F. The mechanism of haem catabolism. Bilirubin formation in living rats by [18O]oxygen labelling. Biochem J. 1978 Feb 15;170(2):297–311. doi: 10.1042/bj1700297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem J. 1976 Oct 1;159(1):23–27. doi: 10.1042/bj1590023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., GREENSTEIN D. S., ROUGHTON F. J. W. The mechanism of catalase action. I. Steady-state analysis. Arch Biochem Biophys. 1952 Jun;37(2):301–321. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- Hatzikonstantinou H., Brown S. B. Catalase model systems. Decomposition of hydrogen peroxide catalysed by mesoferrihaem, deuteroferrihaem, coproferrihaem and haematoferrihaem. Biochem J. 1978 Sep 15;174(3):893–900. doi: 10.1042/bj1740893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. J. Letter: Polymerization of glutaraldehyde at fixative pH. J Histochem Cytochem. 1974 Sep;22(9):911–913. doi: 10.1177/22.9.911. [DOI] [PubMed] [Google Scholar]
- Jones P., Prudhoe K., Robson T. Oxidation of deuteroferrihaem by hydrogen peroxide. Biochem J. 1973 Oct;135(2):361–365. doi: 10.1042/bj1350361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H. C., Davies D. M., King M. J., Jones P. Pre-steady-state kinetics of intermediate formation in the deuteroferriheme-hydrogen peroxide system. Biochemistry. 1977 Aug 9;16(16):3543–3549. doi: 10.1021/bi00635a007. [DOI] [PubMed] [Google Scholar]
- King R. F., Brown S. B. The mechanism of haem catabolism. A study of haem breakdown in spleen microsomal fraction and in a model system by 18O labelling and metal substitution. Biochem J. 1978 Jul 15;174(1):103–109. doi: 10.1042/bj1740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe G. H. The degradation of haem by mammals and its excretion as conjugated bilirubin. Essays Biochem. 1972;8:107–148. [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H., Pimstone N. R., Trager W. F., Cooper D. Y., Schmid R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry. 1972 Apr 25;11(9):1716–1720. doi: 10.1021/bi00759a029. [DOI] [PubMed] [Google Scholar]


