Abstract
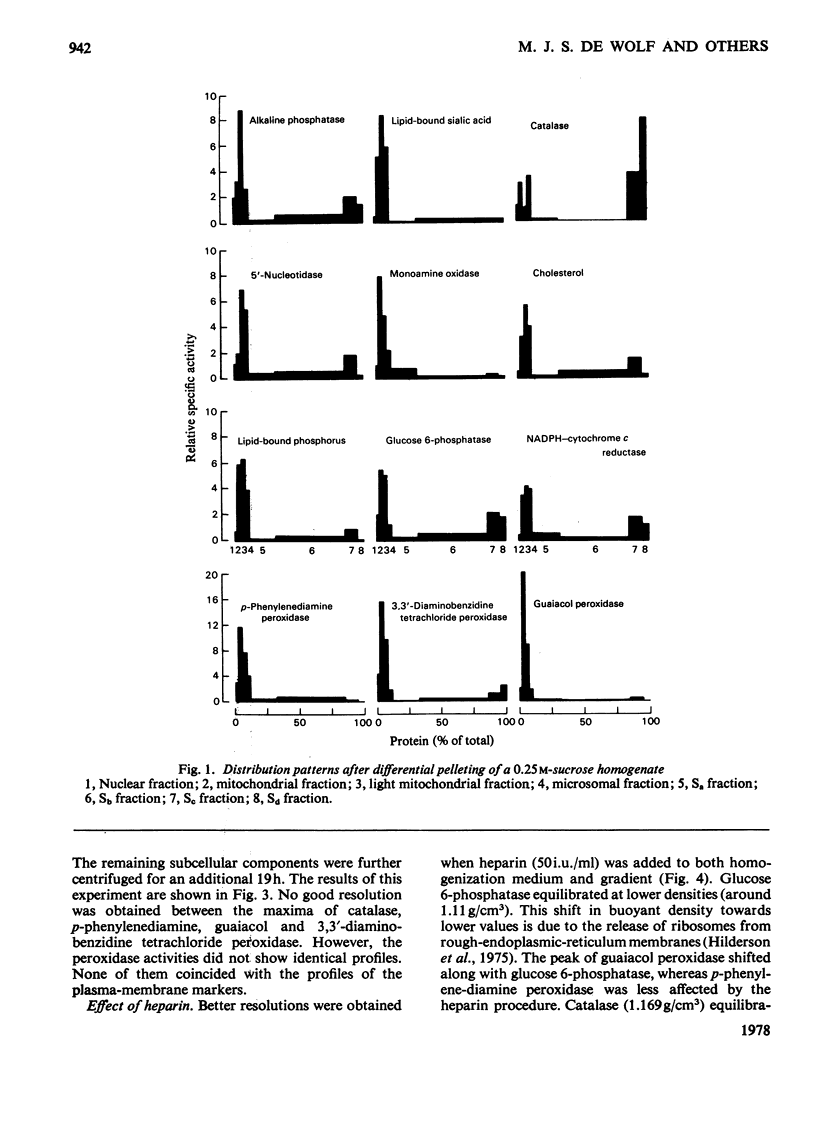
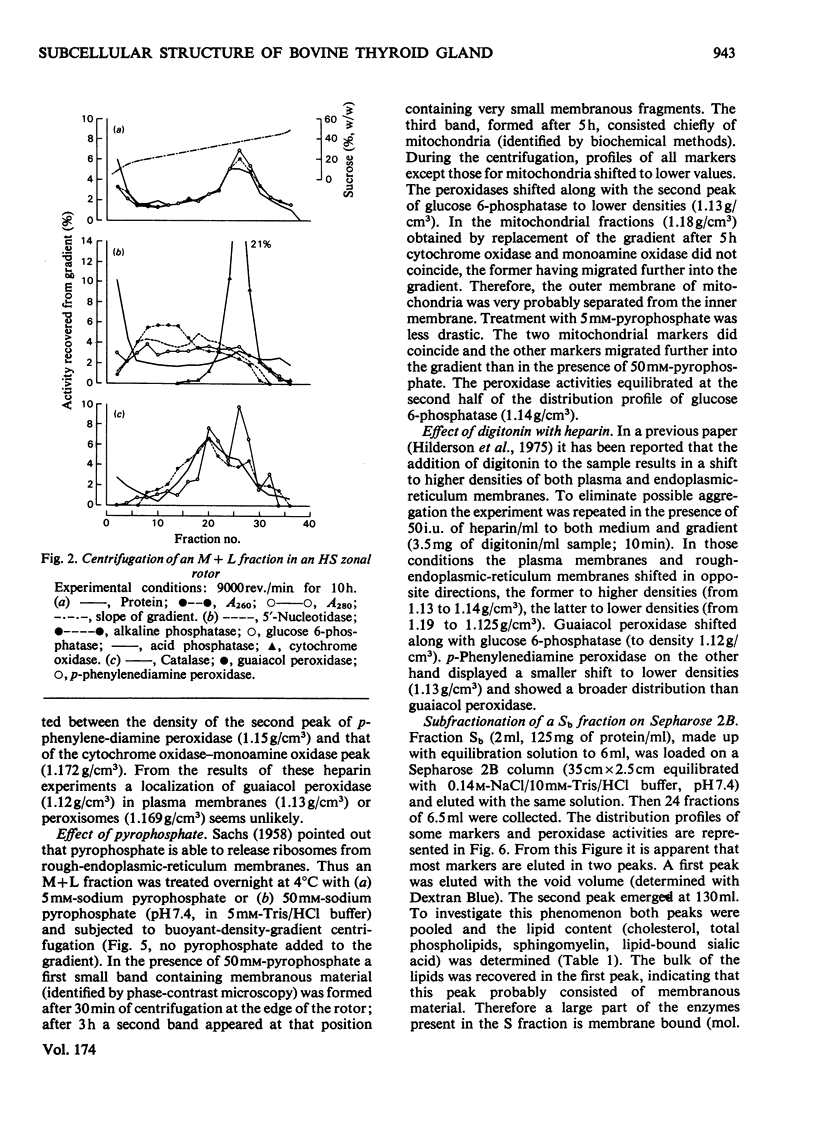
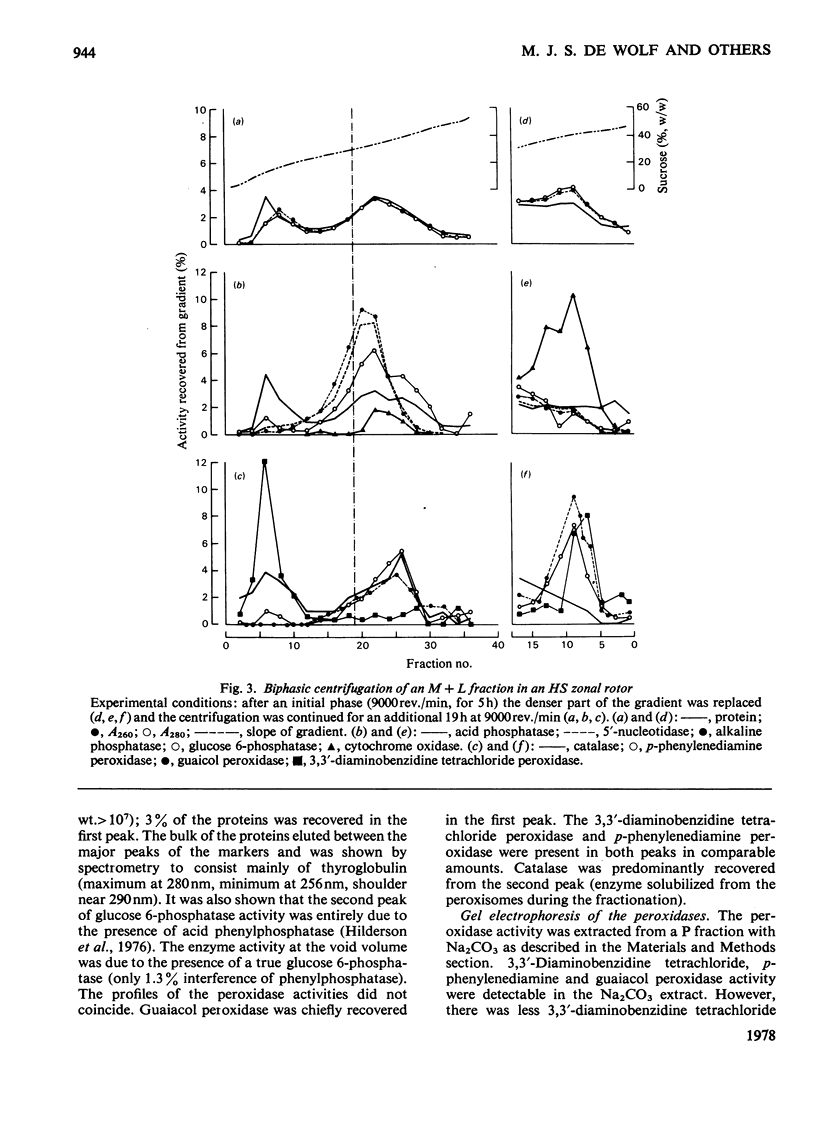
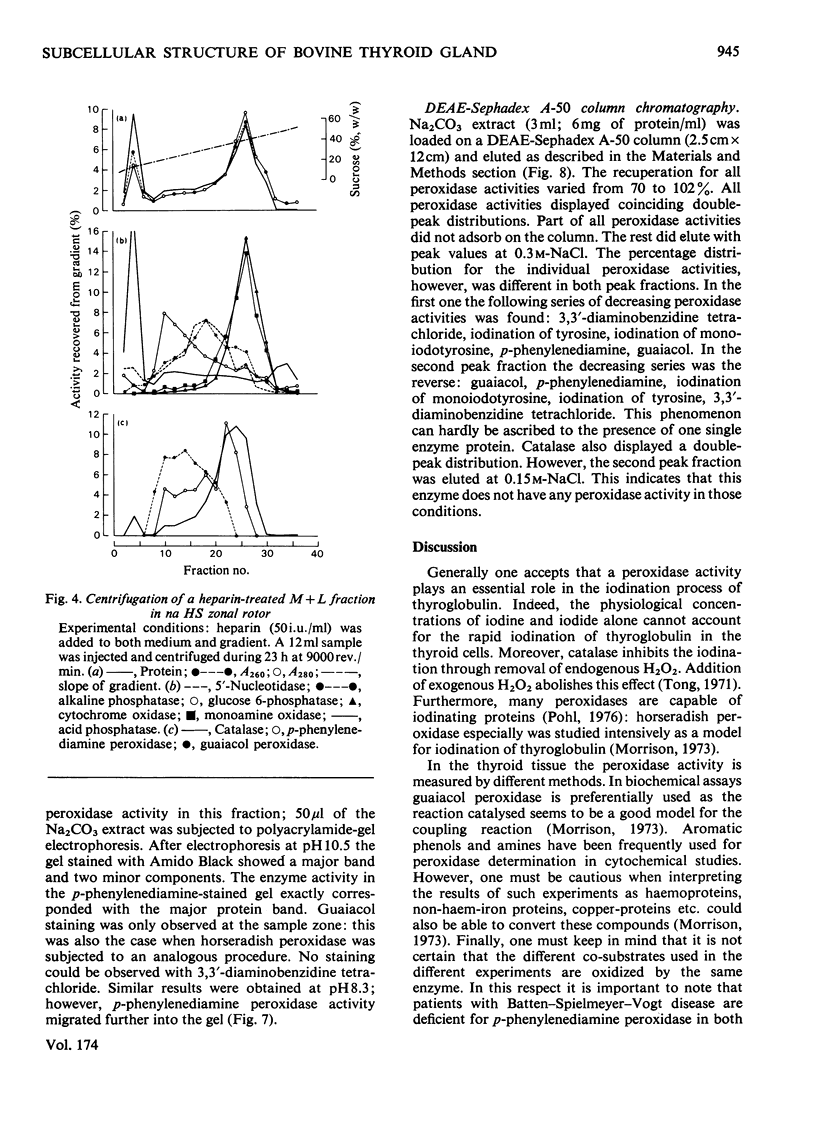
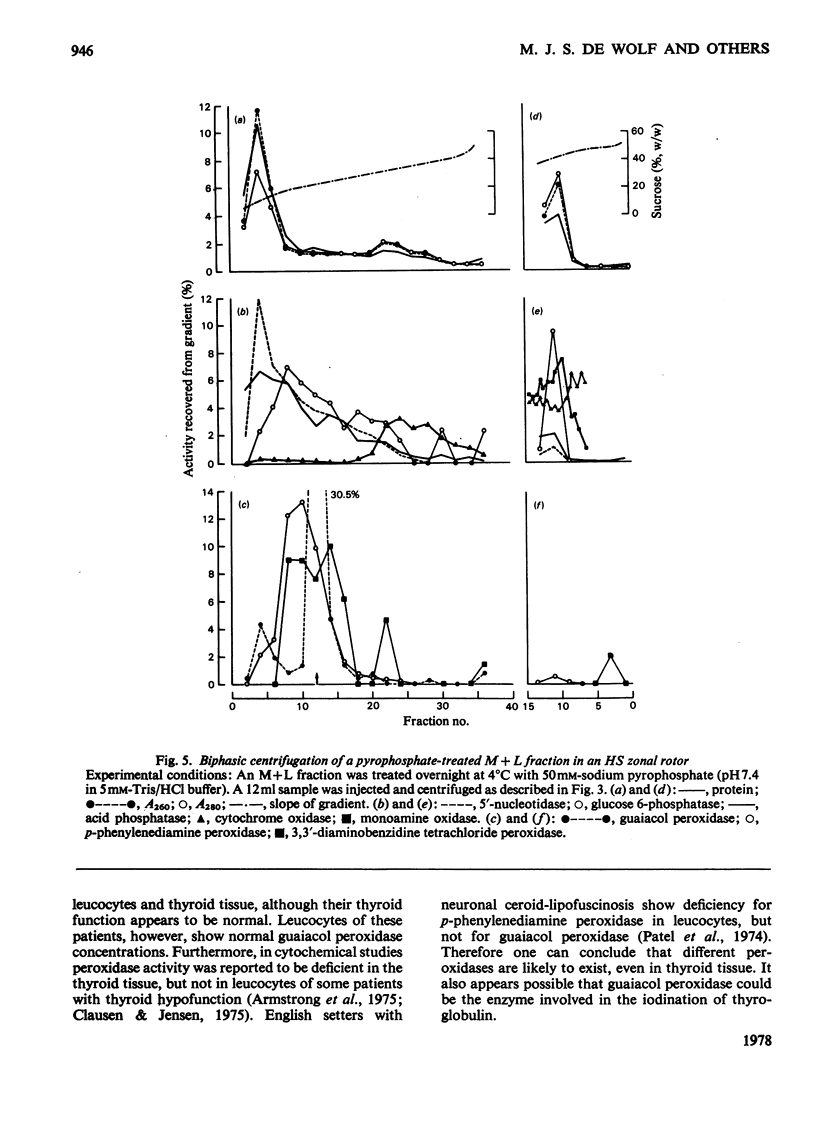
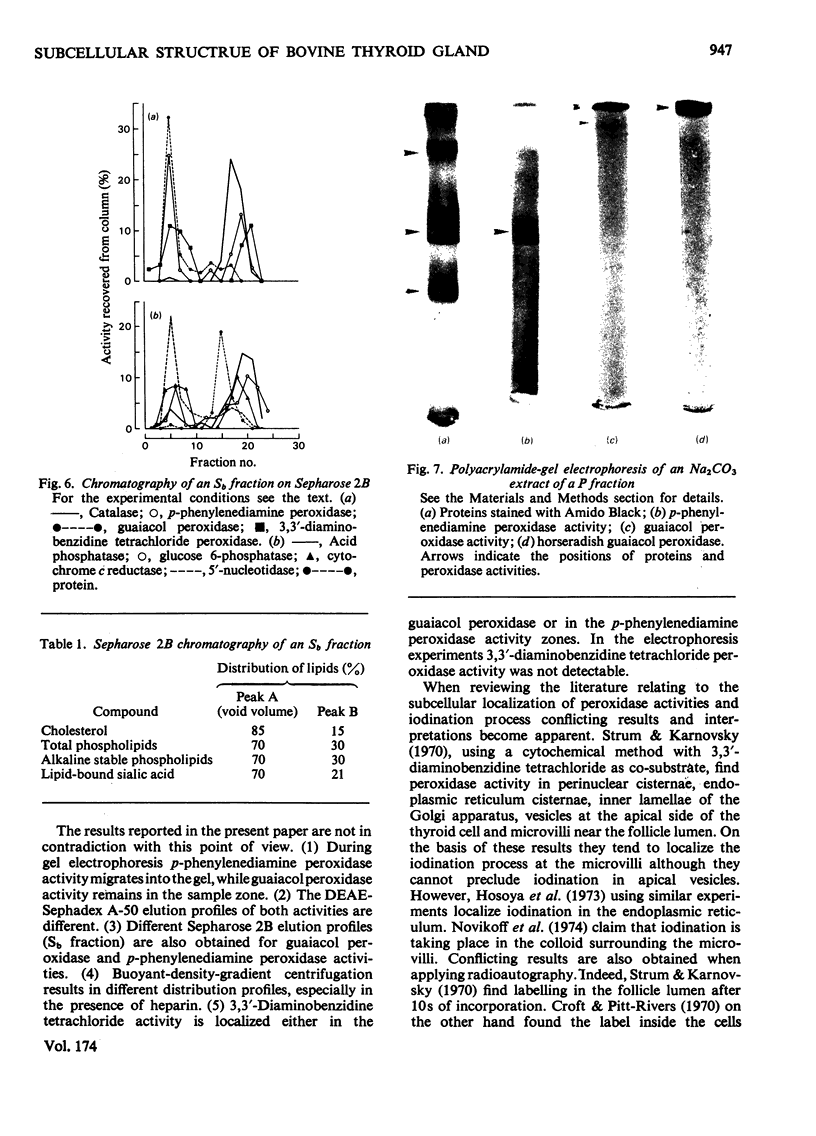
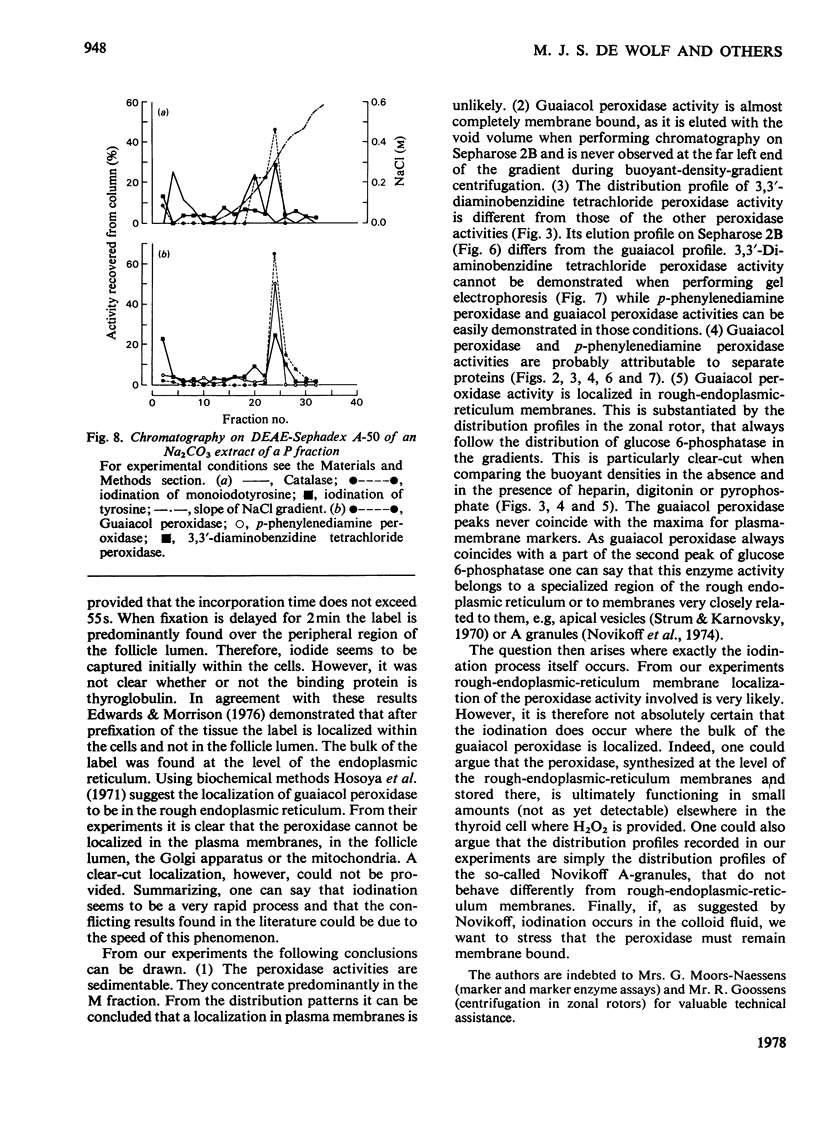
1. After differential pelleting of bovine thyroid tissue the highest relative specific activities for plasma membrane markers are found in the L fraction whereas those for peroxidase activities (p-phenylenediamine, guaiacol and 3,3'-diaminobenizidine tetrachloride peroxidases) are found in the M fraction. 2. When M + L fractions were subjected to buoyant-density equilibration in a HS zonal rotor all peroxidases show different profiles. The guaiacol peroxidase activity always follows the distribution of glucose 6-phosphatase. 3. When a Sb fraction is subjected to Sepharose 2B chromatography three major peaks are obtained. The first, eluted at the void volume, consists of membranous material and contains most of the guaiacol peroxidase activity. Most of the protein (probably thyroglobulin) is eluted with the second peak. Solubilized enzymes are recovered in the third peak. 4. p-Phenylenediamine peroxidase activity penetrates into the gel on polyacrylamidegel electrophoresis, whereas guaiacol peroxidase activity remains at the sample zone. 5. DEAE-Sephadex A-50 chromatography resolves the peroxidase activities into two peaks, displaying different relative amounts of the different enzymic activities in each peak. 6. The peroxidase activities may be due to the presence of different proteins. A localization of guaiacol peroxidase in rough-endoplasmic-reticulum membranes (or in membranes related to them) seems very likely.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., NORTON W. T., KATZMAN R. STUDY OF IONIC STRUCTURES IN PHOSPHOLIPIDS BY INFRARED SPECTRA. J Biol Chem. 1965 Jun;240:2389–2395. [PubMed] [Google Scholar]
- Armstrong D., VanWormer D. E., Neville H., Dimmitt S., Clingan F. Thyroid peroxidase deficiency in Batten-Spielmeyer-Vogt disease. Arch Pathol. 1975 Aug;99(8):430–435. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Clausen J., Jensen G. E. Acid proteinase and peroxidase activity in spielmeyer-Vogt's syndrome (Batten's syndrome-Stengel's syndrome). Clin Chim Acta. 1975 Dec 15;65(3):283–289. doi: 10.1016/0009-8981(75)90253-3. [DOI] [PubMed] [Google Scholar]
- Croft C. J., Pitt-Rivers R. Radioautographic studies of the initial site of formation of protein-bound iodine in the rat thyroid gland. Biochem J. 1970 Jun;118(2):311–314. doi: 10.1042/bj1180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick W., Hilderson H. Subcellular structure of bovine thyroid. I. Beta-glucuronidase: a marker-enzyme for lysosomal particles. Arch Int Physiol Biochim. 1967 Feb;75(1):1–11. doi: 10.3109/13813456709084913. [DOI] [PubMed] [Google Scholar]
- Edwards H. H., Morrison M. Localization of thyroid peroxidase and the site of iodination in rat thyroid gland. Biochem J. 1976 Aug 15;158(2):477–479. doi: 10.1042/bj1580477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderson H. J., De Wolf M. J., Lagrou A. R., Dierick W. S. Subcellular structure of bovine thyroid gland. A study on bovine thyroid membranes by buoyant-density-gradient centrifugation in a B-XIV zonal rotor. Biochem J. 1975 Dec;152(3):601–607. doi: 10.1042/bj1520601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderson H. J., Lagrou A., Dierick W. The nuclear lipids of bovine hypertrophic thyroid. Biochim Biophys Acta. 1974 Mar 28;337(3):385–389. doi: 10.1016/0005-2760(74)90113-1. [DOI] [PubMed] [Google Scholar]
- Hosoya T., Matsukawa S. Biochemical studies on the peroxidase activity in the normal and hyperplastic thyroids of rats. Endocrinol Jpn. 1975 Feb;22(1):25–34. doi: 10.1507/endocrj1954.22.25. [DOI] [PubMed] [Google Scholar]
- Hosoya T., Matsukawa S., Nagai Y. Localization of peroxidase and other microsomal enzymes in thyroid cells. Biochemistry. 1971 Aug 3;10(16):3086–3093. doi: 10.1021/bi00792a016. [DOI] [PubMed] [Google Scholar]
- KIND P. R., KING E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954 Nov;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagrou A., Hilderson H. J., de Wolf M., Dierick W. Bovine thyroid gangliosides: distribution pattern after differential pelleting. Arch Int Physiol Biochim. 1974 Oct;82(4):733–736. doi: 10.3109/13813457409072325. [DOI] [PubMed] [Google Scholar]
- Morrison M. Thyroid peroxidase-catalyzed iodination and coupling reactions and their control. Ann N Y Acad Sci. 1973;212:175–182. doi: 10.1111/j.1749-6632.1973.tb47595.x. [DOI] [PubMed] [Google Scholar]
- Mushahwar I. K., Oliner L., Schulz A. R. Bovine thyroid microsomal monoamine oxidase. Can J Biochem. 1972 Oct;50(10):1035–1047. doi: 10.1139/o72-144. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Davidson B., Armstrong A., Maloof F. Partial purification and some properties of thyroid peroxidase solubilized by alkaline treatment. Prep Biochem. 1973;3(5):495–508. doi: 10.1080/00327487308061531. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Ma M., Shin W. Y., Quintana N. Cytochemical studies of secretory and other granules associated with the endoplasmic reticulum in rat thyroid epithelial cells. Adv Cytopharmacol. 1974;2:349–368. [PubMed] [Google Scholar]
- Patel V., Koppang N., Patel B., Zeman W. P-phenylenediamine-mediated peroxidase deficiency in English setters with neuronal ceroid-lipofuscinosis. Lab Invest. 1974 Mar;30(3):366–368. [PubMed] [Google Scholar]
- Pohl S. L. Iodination of liver plasma membranes using lactoperoxidase: effects on adenylate cyclase activity. Proc Soc Exp Biol Med. 1976 Jul;152(3):327–329. doi: 10.3181/00379727-152-39389. [DOI] [PubMed] [Google Scholar]
- SACHS H. The effect of pyrophosphate on the amino acid incorporating system of rat liver microsomes. J Biol Chem. 1958 Sep;233(3):650–656. [PubMed] [Google Scholar]
- Strum J. M., Karnovsky M. J. Cytochemical localization of endogenous peroxidase in thyroid follicular cells. J Cell Biol. 1970 Mar;44(3):655–666. doi: 10.1083/jcb.44.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]