Abstract
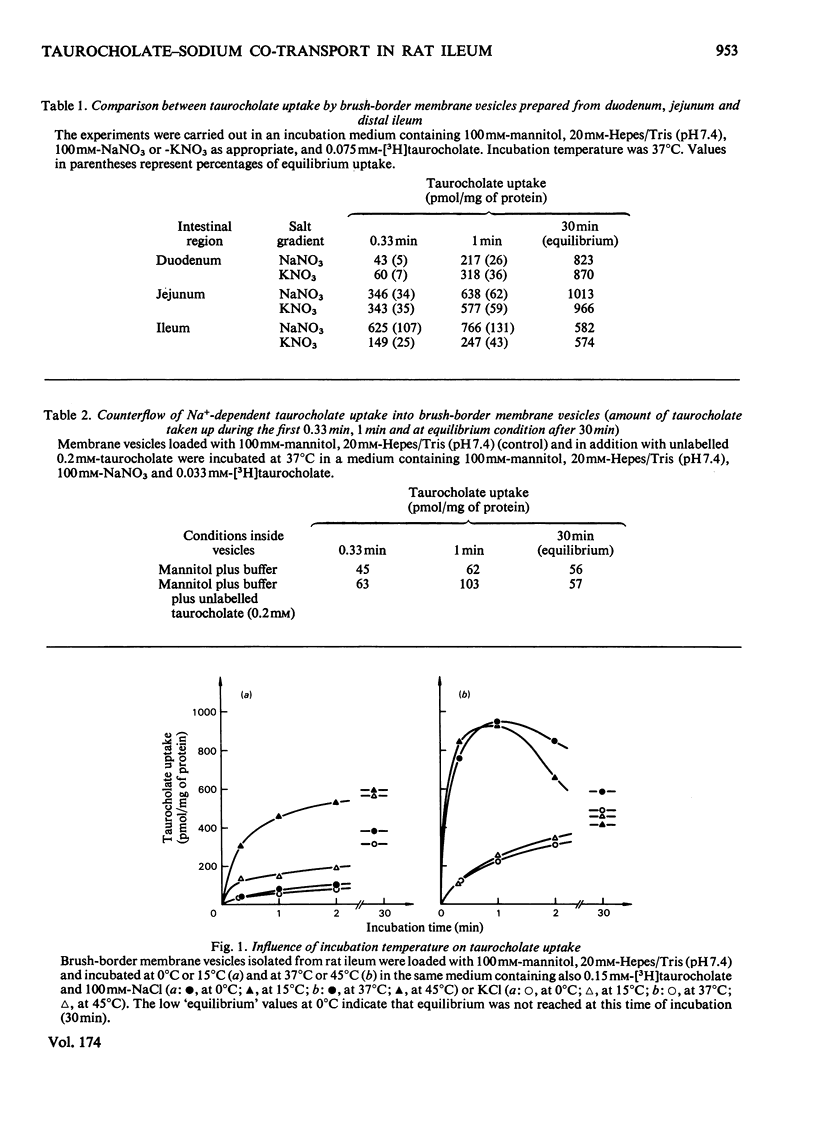
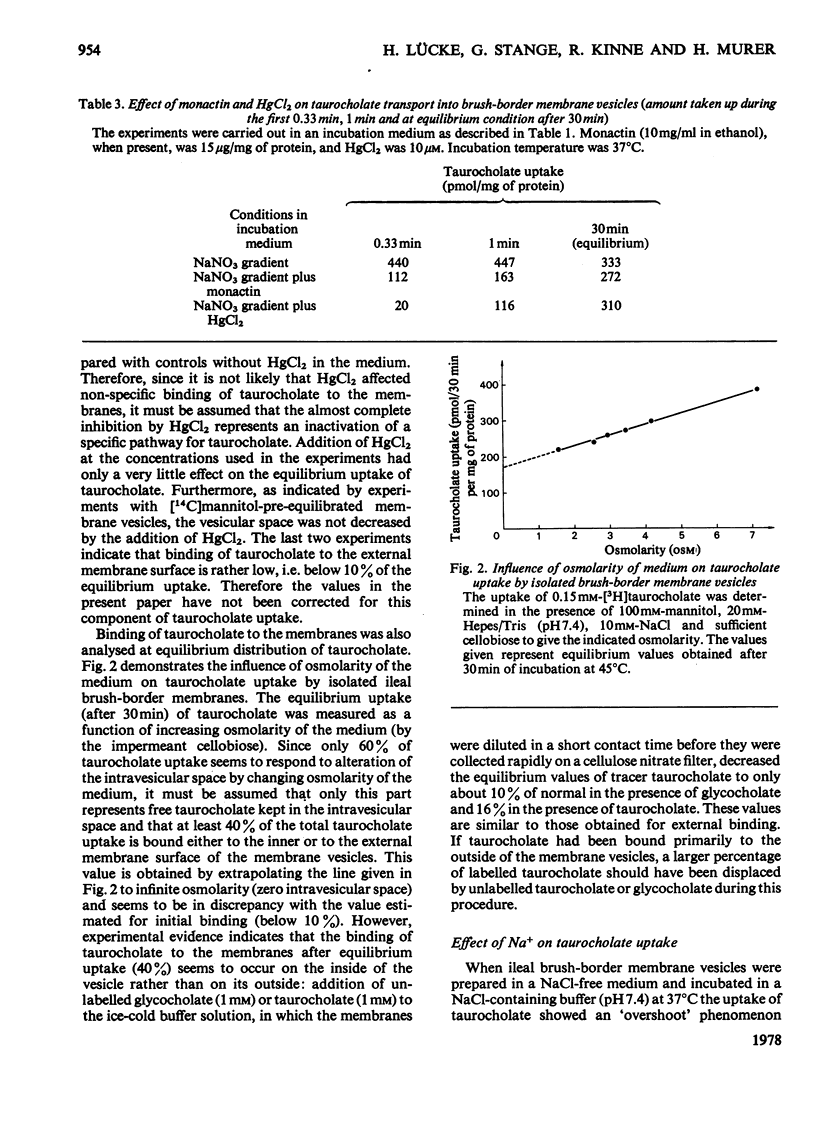
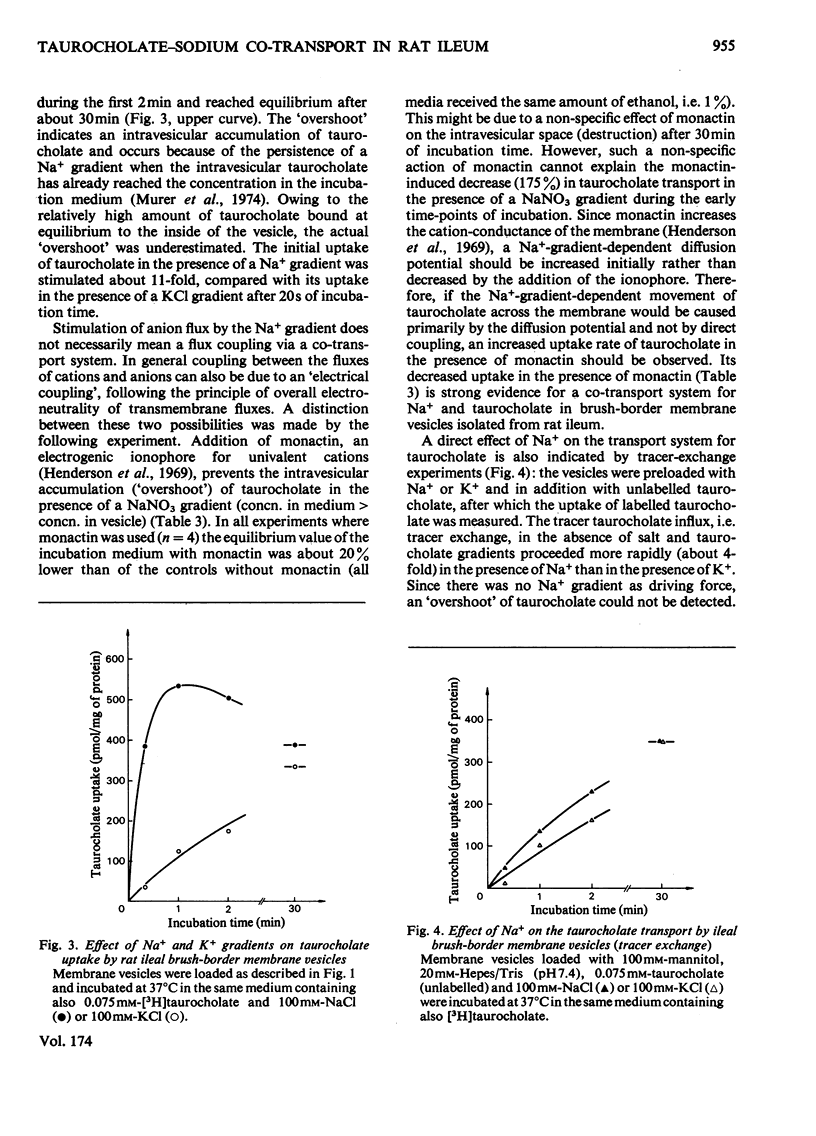
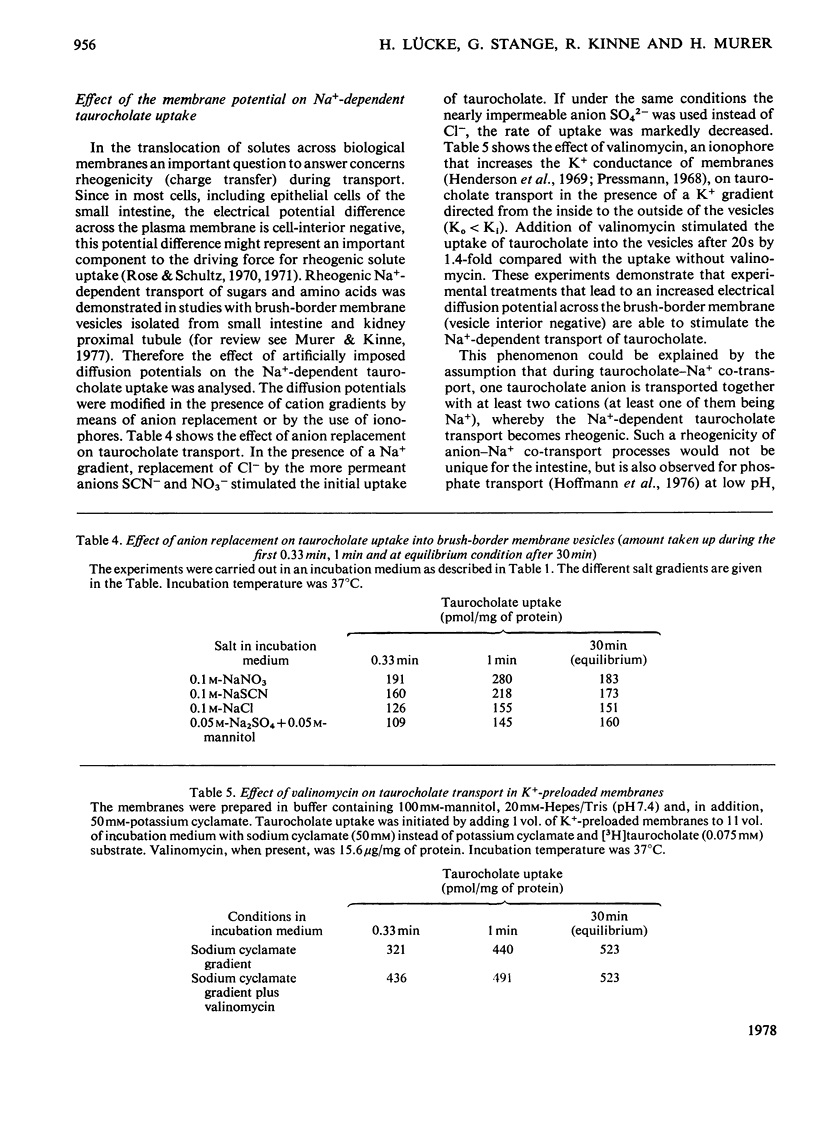
Uptake of taurocholate into brush-border membrane vesicles isolated from rat small intestine by a Ca2+ -precipitation method was investigated by using a rapid-filtration technique. Uptake of taurocholate by ileal brush-border membranes consisted of three phenomena: binding to the outside of the vesicles, transfer across the vesicle membrane and binding to the intravesicular compartment. The transport of taurocholate across the brush-border membranes was stimulated in the presence of Na+ compared with the presence of K+; stimulation was about 11-fold in the presence of a NaCl gradient (Nao>Nai), where the subscripts refer to `outside' and `inside' respectively, and 4-fold under equilibrium conditions for Na+ (Nao=Nai). In the presence of a Na+ gradient a typical `overshoot' phenomenon was observed. Membranes preloaded with unlabelled taurocholate showed an accelerated entry of labelled taurocholate (tracer exchange) in the presence of Na+ compared with the presence of K+. The stimulation by Na+ was observed only in membrane preparations from the ileum. Addition of monactin, an ionophore for univalent cations, decreased the Na+-gradient-driven taurocholate uptake. The Na+-dependent taurocholate transport showed saturation kinetics and the phenomenon of counterflow and was inhibited by glycocholate. Other cations such as Li+, Rb+ and Cs+ could not replace Na+ in its stimulatory action. When the electrical potential difference across the vesicle membrane was altered by establishing different diffusion potentials (anion replacement; K+ gradient±valinomycin) a more-negative potential inside stimulated Na+-dependent taurocholate transport. These data demonstrate the presence of a rheogenic (potential sensitive) Na+–taurocholate co-transport system in ileal brush-border membranes and support the hypothesis that the reabsorption of bile acids in the ileum is a secondary active uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berner W., Kinne R., Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J. 1976 Dec 15;160(3):467–474. doi: 10.1042/bj1600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner W., Kinne R. Transport of p-aminohippuric acid by plasma membrane vesicles isolated from rat kidney cortex. Pflugers Arch. 1976 Feb 24;361(3):269–277. doi: 10.1007/BF00587292. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- GLASSER J. E., WEINER I. M., LACK L. COMPARATIVE PHYSIOLOGY OF INTESTINAL TAUROCHOLATE TRANSPORT. Am J Physiol. 1965 Feb;208:359–362. doi: 10.1152/ajplegacy.1965.208.2.359. [DOI] [PubMed] [Google Scholar]
- HOLT P. R. INTESTINAL ABSORPTION OF BILE SALTS IN THE RAT. Am J Physiol. 1964 Jul;207:1–7. doi: 10.1152/ajplegacy.1964.207.1.1. [DOI] [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Krag E., Phillips S. F. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974 Jun;53(6):1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lack L., Walker J. T., Hsu C. Y. Taurocholate uptake by membrane vesicles prepared from ileal brush borders. Life Sci. 1977 May 1;20(9):1607–1611. doi: 10.1016/0024-3205(77)90455-6. [DOI] [PubMed] [Google Scholar]
- Lack L., Weiner I. M. Intestinal bile salt transport: structure-activity relationships and other properties. Am J Physiol. 1966 May;210(5):1142–1152. doi: 10.1152/ajplegacy.1966.210.5.1142. [DOI] [PubMed] [Google Scholar]
- Lúcke H., Berner W., Menge H., Murer H. Sugar transport by brush border membrane vesicles isolated from human small intestine. Pflugers Arch. 1978 Mar 20;373(3):243–248. doi: 10.1007/BF00580831. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne-Saffran E., Kinne R. Glucose transport in isolated brush-border and lateral-basal plasma-membrane vesicles from intestinal epithelial cells. Biochim Biophys Acta. 1974 Apr 29;345(2):170–179. doi: 10.1016/0005-2736(74)90256-9. [DOI] [PubMed] [Google Scholar]
- PLAYOUST M. R., ISSELBACHER K. J. STUDIES ON THE TRANSPORT AND METABOLISM OF CONJUGATED BILE SALTS BY INTESTINAL MUCOSA. J Clin Invest. 1964 Mar;43:467–476. doi: 10.1172/JCI104932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff E. R., Small N. C., Dietschy J. M. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972 Jun;51(6):1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest. 1972 Dec;51(12):3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Treanor L. L. Characterization of bile acid binding to rat intestinal brush border membranes. J Membr Biol. 1977 May 12;33(3-4):213–230. doi: 10.1007/BF01869517. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Treanor L. L. Characterization of the passive and active transport mechanisms for bile acid uptake into rat isolated intestinal epithelial cells. Biochim Biophys Acta. 1975 Oct 6;406(2):280–293. doi: 10.1016/0005-2736(75)90010-3. [DOI] [PubMed] [Google Scholar]


