Abstract
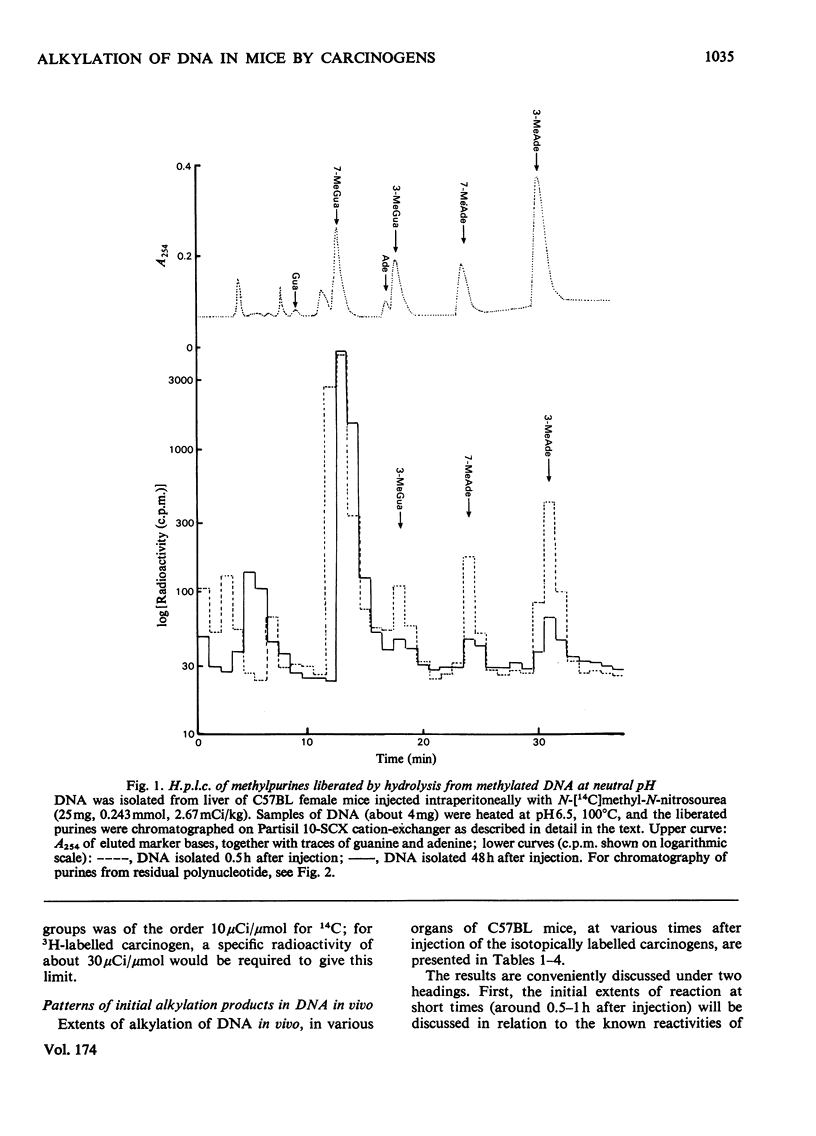
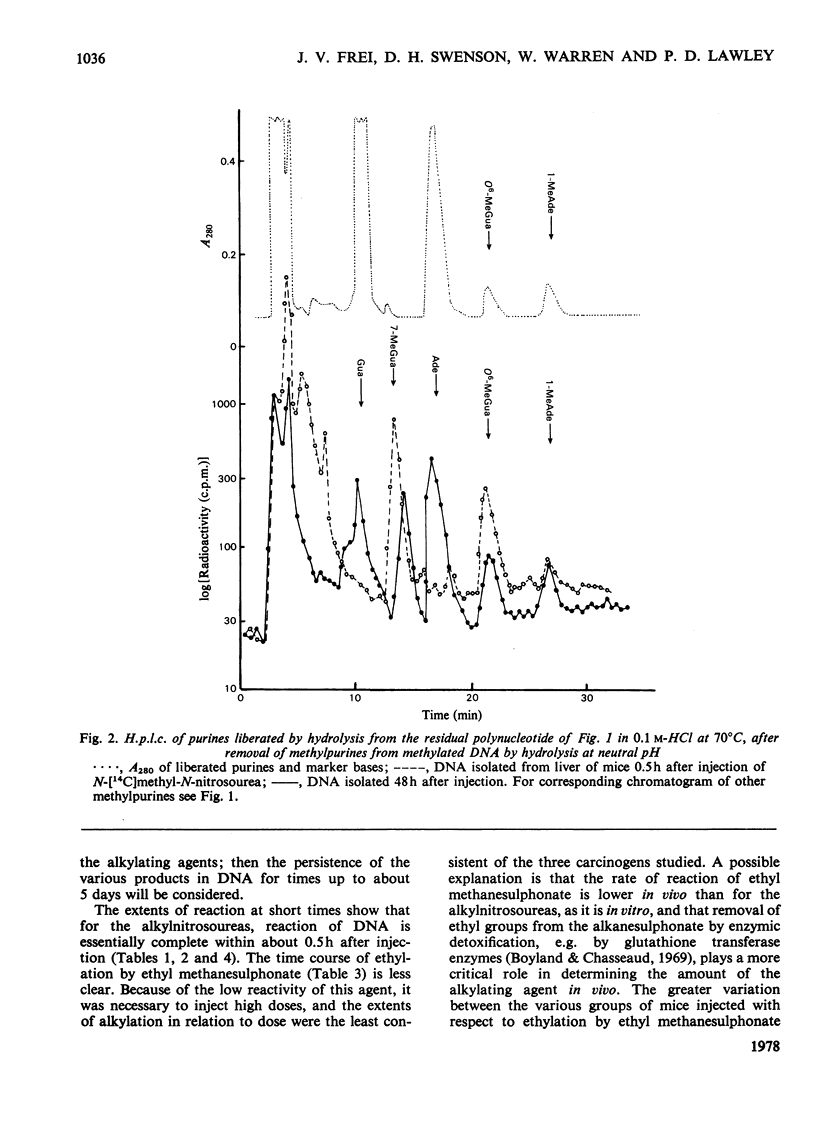
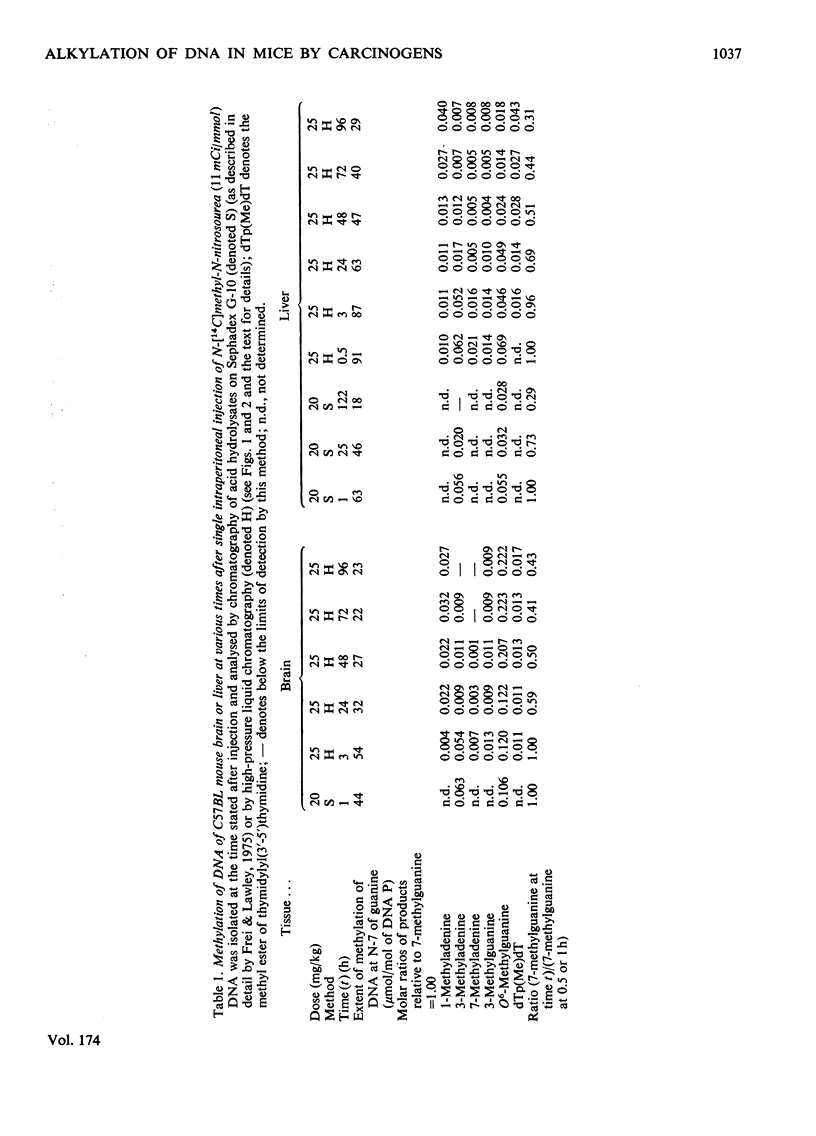
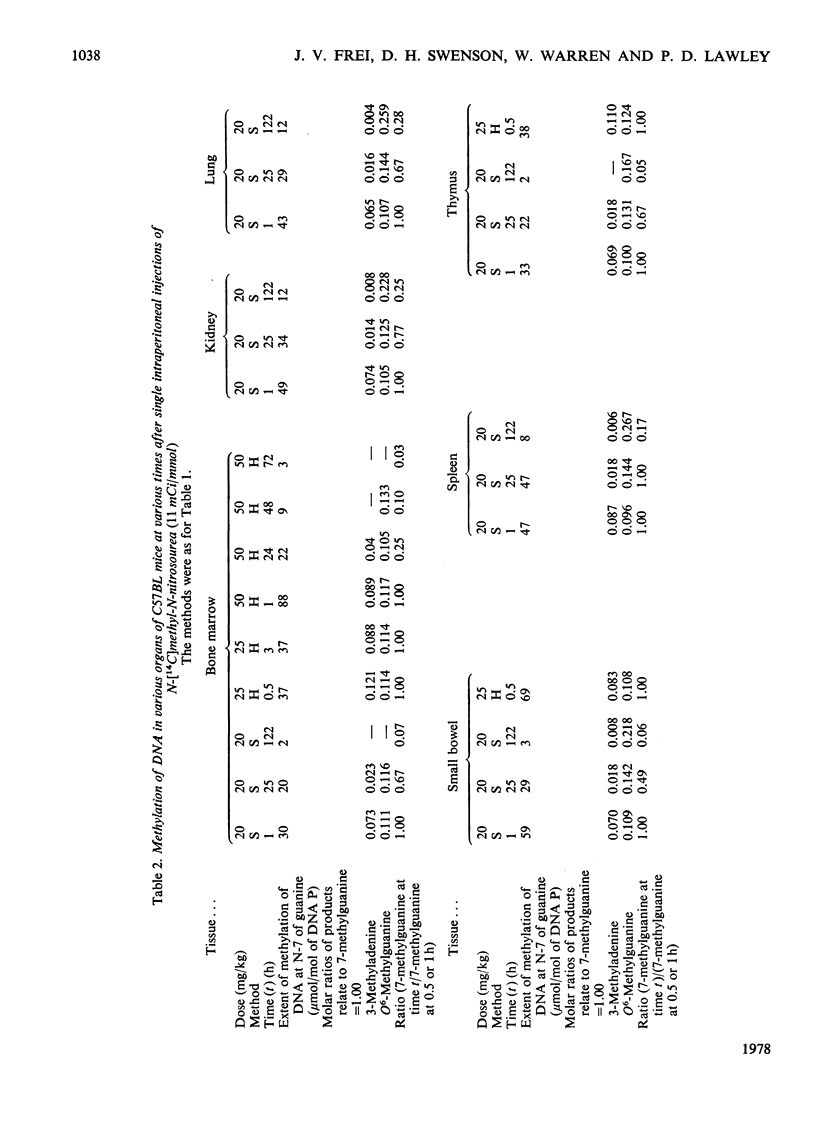
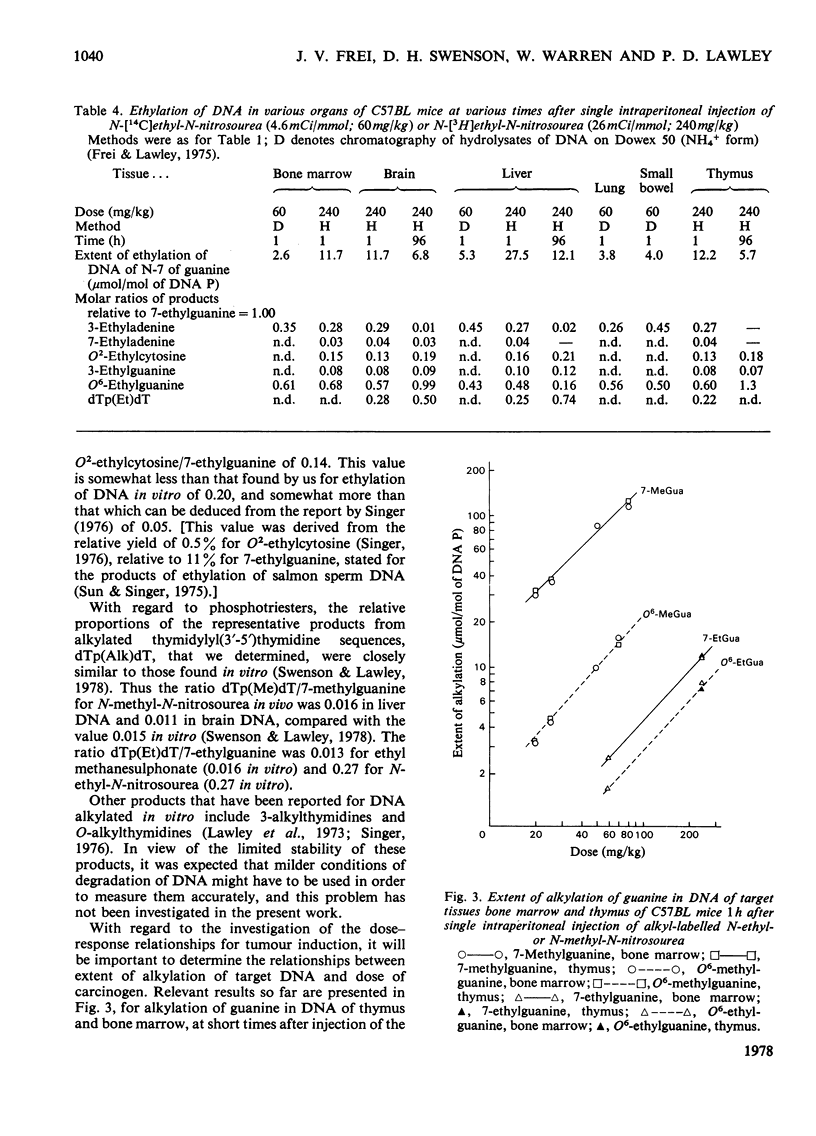
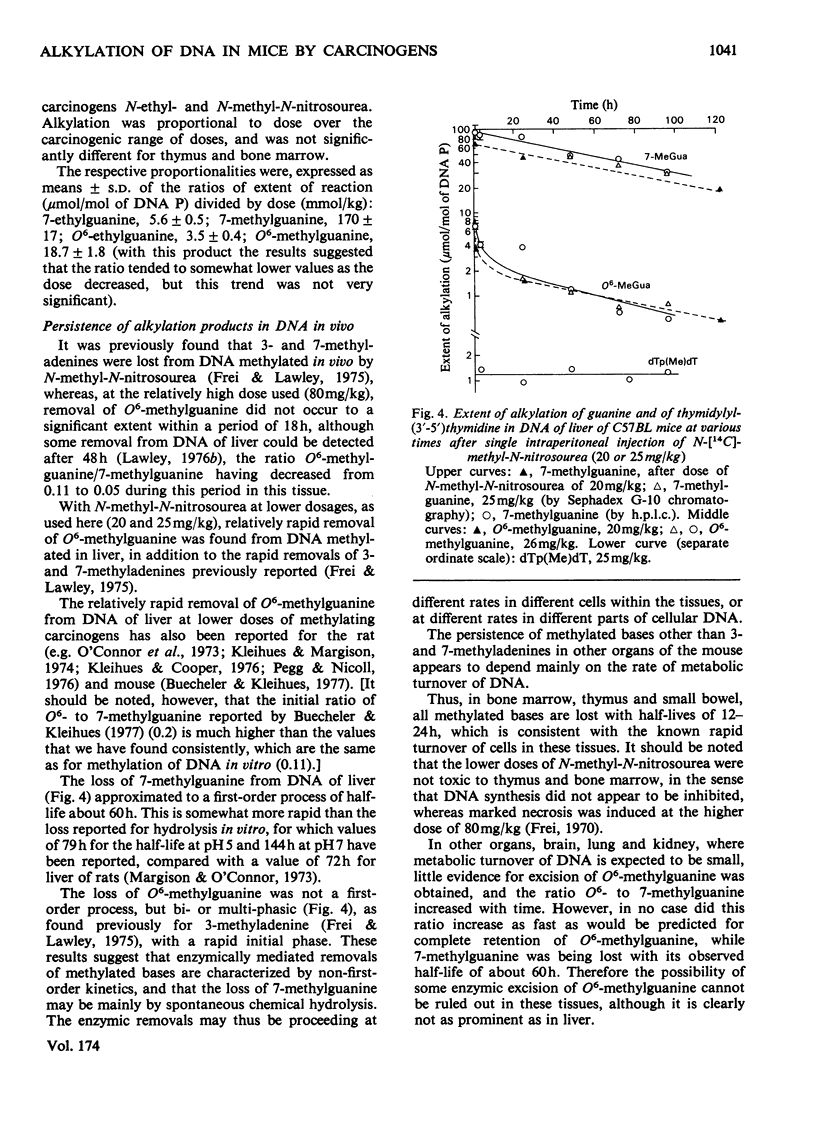
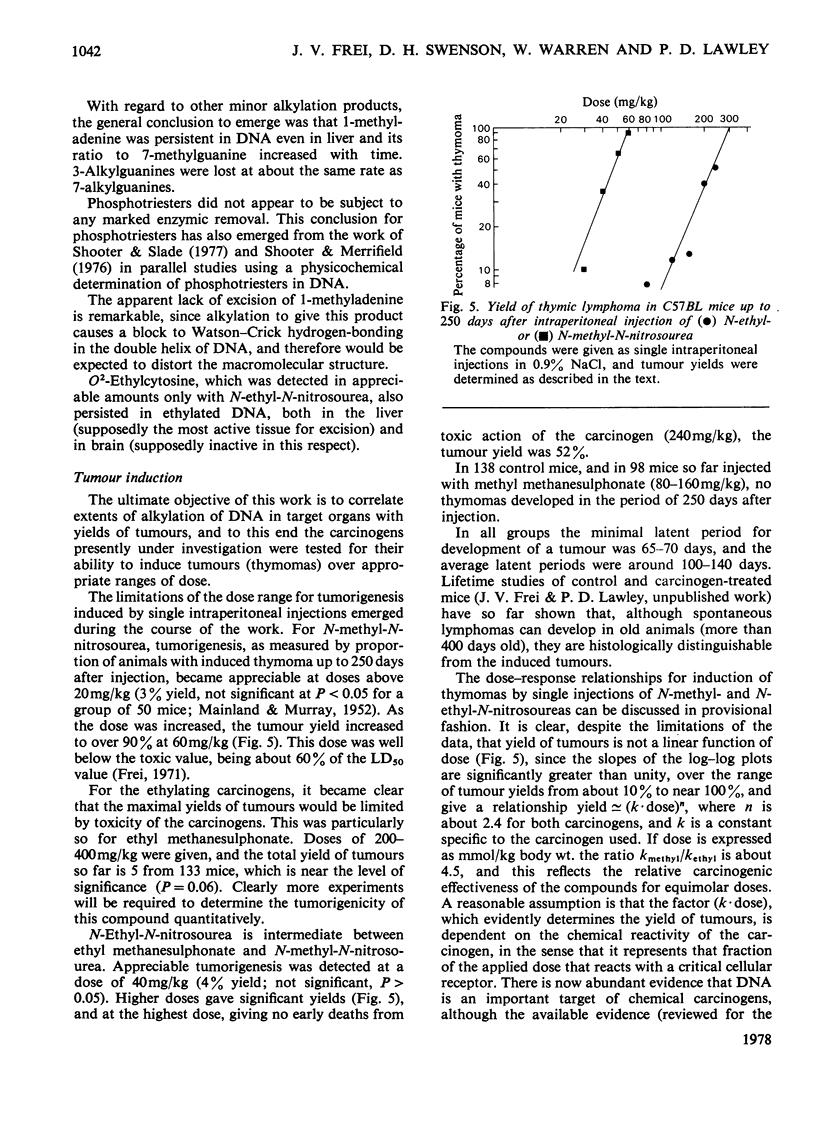
1. Methods were developed for analysis of alkylpurines, O2-alkylcytosines, and representative phosphotriesters [alkyl derivatives of thymidylyl(3'-5')thymidine], in DNA alkylated in vivo, using high-pressure liquid chromatography. 2. The patterns of alkylation products in DNA in vivo at short times were closely similar to those found for reactions in vitro. Alkylation by the nitrosoureas was complete in vivo within 1 h, but with ethyl methanesulphonate was maximal at 2--4h. 3. The time course of persistence of alkylation products in vivo was determined for several tissues. In addition to the rapid loss of 3- and 7-alkyladenines reported previously for all tissues, a relatively rapid loss of O6-alkylguanines from DNA of liver was found which was more rapid at lower doses. In brain, lung and kidney, excision of O6-alkylguanine was much less marked, but was not entirely excluded by the data. In thymus, bone marrow and small bowel, all alkylated bases were lost with half-lives of 12--24h, at non-cytotoxic doses of alkylation. 4. No evidence for any marked excision of other minor products from alkylated DNA in vivo was found; thus 1-methyladenine, O2-ethylcytosine (found in appreciable amount only with N-ethyl-N-nitrosourea), 3-methylguanine, and dTp(Alk)dT persisted in alkylated DNA, including DNA of liver. 5. The induction of thymic lymphoma was determined over the range of single doses by intraperitoneal injection up to about 60% of the LD50 values, and related to the extent of alkylation of target tissues thymus and bone marrow. With N-methyl-N-nitrosourea over 90% tumour yield was attained at 60 mg/kg, and with N-ethyl-N-nitrosourea up to 52% at 240 mg/kg, but with ethyl methanesulphonate at up to 400 mg/kg only a few per cent of tumours were obtained. 6. The carcinogenic effectiveness of the agents was positively correlated with the extents of alkylation of guanine in DNA of target tissues at the O-6 atom. On the basis that at doses giving equal carcinogenic response these extents of alkylation would be equal, the chemical analyses showed that the ratio of equipotent doses to that for N-methyl-N-nitrosourea would be, for N-ethyl-N-nitrosourea, 5.3 for ethyl methanesulphonate about 21, and for methyl methanesulphonate [Frei & Lawley (1976) Chem.-Biol. Interact. 13, 215--222] about 144. These predictions were in reasonably good agreement with the observed dose-response data for these agents.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Buecheler J., Kleihues P. Excision of O6-methylguanine from DNA of various mouse tissues following a single injection of N-methyl-Nitrosourea. Chem Biol Interact. 1977 Mar;16(3):325–333. doi: 10.1016/0009-2797(77)90112-0. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Lawley P. D. Methylation of DNA in various organs of C57B1 mice by a carcinogenic dose of N-methyl-N-nitrosourea and stabiltty of some methylation products up to 18 hours. Chem Biol Interact. 1975 Jun;10(6):413–427. doi: 10.1016/0009-2797(75)90072-1. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Lawley P. D. Tissue distribution and mode of DNA methylation in mice by methyl methanesulphonate and N-methyl-N' -nitro-N-nitrosoguanidine: lack of thymic lymphoma induction and low extent of methylation of target tissue DNA at 0-6 of guanine. Chem Biol Interact. 1976 Jun;13(3-4):215–222. doi: 10.1016/0009-2797(76)90075-2. [DOI] [PubMed] [Google Scholar]
- Frei J. V. Toxicity, tissue changes, and tumor induction in inbred Swiss mice by methylnitrosamine and -amide compounds. Cancer Res. 1970 Jan;30(1):11–17. [PubMed] [Google Scholar]
- Frei J. V. Tumour induction by low molecular weight alkylating agents. Chem Biol Interact. 1971 Apr;3(2):117–121. doi: 10.1016/0009-2797(71)90091-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Molecular and cellular mechanisms associated with pulse-carcinogenesis in the rat nerbous system by ethyinitrosourea: ethylation of nucleic acids and elimination rates of ethylated bases from the DNA of different tissues. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;82(1):37–64. doi: 10.1007/BF00304382. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Frei J. V. Effects of dose and schedule of methylnitrosourea on incidence of malignant lymphoma in adult female mice. J Natl Cancer Inst. 1970 Aug;45(2):335–339. [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Cooper H. K. Repair excision of alkylated bases from DNA in vivo. Oncology. 1976;33(2):86–88. doi: 10.1159/000225111. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976 Jan 15;259(5539):153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- Kuśmierek J. T., Singer B. Reaction of diazoalkanes with 1-substituted 2, 4-dioxopyrimidines. Formation of O2, N-3 and O4-alkyl products. Nucleic Acids Res. 1976 Apr;3(4):989–1000. doi: 10.1093/nar/3.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Martin C. N. Molecular mechanisms in alkylation mutagenesis. Induced reversion of bacteriophage T4rII AP72 by ethyl methanesulphonate in relation to extent and mode of ethylation of purines in bacteriophage deoxyribonucleic acid. Biochem J. 1975 Jan;145(1):85–91. doi: 10.1042/bj1450085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Jarman M. Isolation and identification of products from alkylation of nucleic acids: ethyl- and isopropyl-purines. Biochem J. 1975 Jan;145(1):73–84. doi: 10.1042/bj1450073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Shah S. A., Farmer P. B., Jarman M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem J. 1973 Sep;135(1):193–201. doi: 10.1042/bj1350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D. Reaction of N-methyl-N-nitrosourea (MNUA) with 32P-labelled DNA: evidence for formation of phosphotriesters. Chem Biol Interact. 1973 Aug;7(2):127–130. doi: 10.1016/0009-2797(73)90022-7. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Methylation of DNA by 3H-14C-methyl-labelled N-methyl-N-nitrosourea--evidence for transfer of the intact methyl group. Chem Biol Interact. 1973 Aug;7(2):115–120. doi: 10.1016/0009-2797(73)90020-3. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Methylation of ribonucleic acid by the carcinogens dimethyl sulphate, N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine. Comparisons of chemical analyses at the nucleoside and base levels. Biochem J. 1972 Jun;128(1):117–132. doi: 10.1042/bj1280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Warren W. Specific excision of ethylated purines from DNA of Escherichia coli treated with N-ethyl-N-nitrosourea. Chem Biol Interact. 1975 Jul;11(1):55–57. doi: 10.1016/0009-2797(75)90066-6. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- MAINLAND D., MURRAY I. M. Tables for use in fourfold contingency tests. Science. 1952 Nov 28;116(3022):591–594. doi: 10.1126/science.116.3022.591. [DOI] [PubMed] [Google Scholar]
- MacGibbon A. K., Buckley P. D., Blackwell L. F. Evidence for two-step binding of reduced nicotinamide-adenine dinucleotide to aldehyde dehydrogenase. Biochem J. 1977 Sep 1;165(3):455–462. doi: 10.1042/bj1650455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Methylated purines in the deoxyribonucleic acid of various Syrian-golden-hamster tissues after administration of a hepatocarcinogenic dose of dimethylnitrosamine. Biochem J. 1976 Sep 1;157(3):627–634. doi: 10.1042/bj1570627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., O'Connor P. J. Biological implications of the instability of the N-glycosidic bone of 3-methyldeoxyadenosine in DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):349–356. doi: 10.1016/0005-2787(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Capps M. J., Craig A. W. Comparative studies of the hepatocarcinogen N,N-dimethylnitrosamine in vivo: reaction sites in rat liver DNA and the significance of their relative stabilities. Br J Cancer. 1973 Feb;27(2):153–166. doi: 10.1038/bjc.1973.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Alkylation of rat liver DNA by dimethylnitrosamine: effect of dosage on O6-methylguanine levels. J Natl Cancer Inst. 1977 Mar;58(3):681–687. doi: 10.1093/jnci/58.3.681. [DOI] [PubMed] [Google Scholar]
- Shooter K. V., Merrifield R. K. An assay for phosphotriester formation in the reaction of alkylating agents with deoxyribosenucleic acid in vitro and in vivo. Chem Biol Interact. 1976 Jun;13(3-4):223–236. doi: 10.1016/0009-2797(76)90076-4. [DOI] [PubMed] [Google Scholar]
- Shooter K. V., Slade T. A. The stability of methyl and ethyl phosphotriesters in DNA in vivo. Chem Biol Interact. 1977 Dec;19(3):353–361. doi: 10.1016/0009-2797(77)90057-6. [DOI] [PubMed] [Google Scholar]
- Shooter K. V. The kinetics of the alkaline hydrolysis of phosphotriesters in DNA. Chem Biol Interact. 1976 May;13(2):151–163. doi: 10.1016/0009-2797(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Sun L., Singer B. The specificity of different classes of ethylating agents toward various sites of HeLa cell DNA in vitro and in vivo. Biochemistry. 1975 Apr 22;14(8):1795–1802. doi: 10.1021/bi00679a036. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Farmer P. B., Lawley P. D. Identification of the methyl phosphotriester of thymidylyl (3',5')thymidine as a product from reaction of DNA with the carcinogen N-methyl-N-nitrosourea. Chem Biol Interact. 1976 Sep;15(1):91–100. doi: 10.1016/0009-2797(76)90131-9. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Lawley P. D. Alkylation of deoxyribonucleic acid by carcinogens dimethyl sulphate, ethyl methanesulphonate, N-ethyl-N-nitrosourea and N-methyl-N-nitrosourea. Relative reactivity of the phosphodiester site thymidylyl(3'-5')thymidine. Biochem J. 1978 Jun 1;171(3):575–587. doi: 10.1042/bj1710575. [DOI] [PMC free article] [PubMed] [Google Scholar]



