Abstract
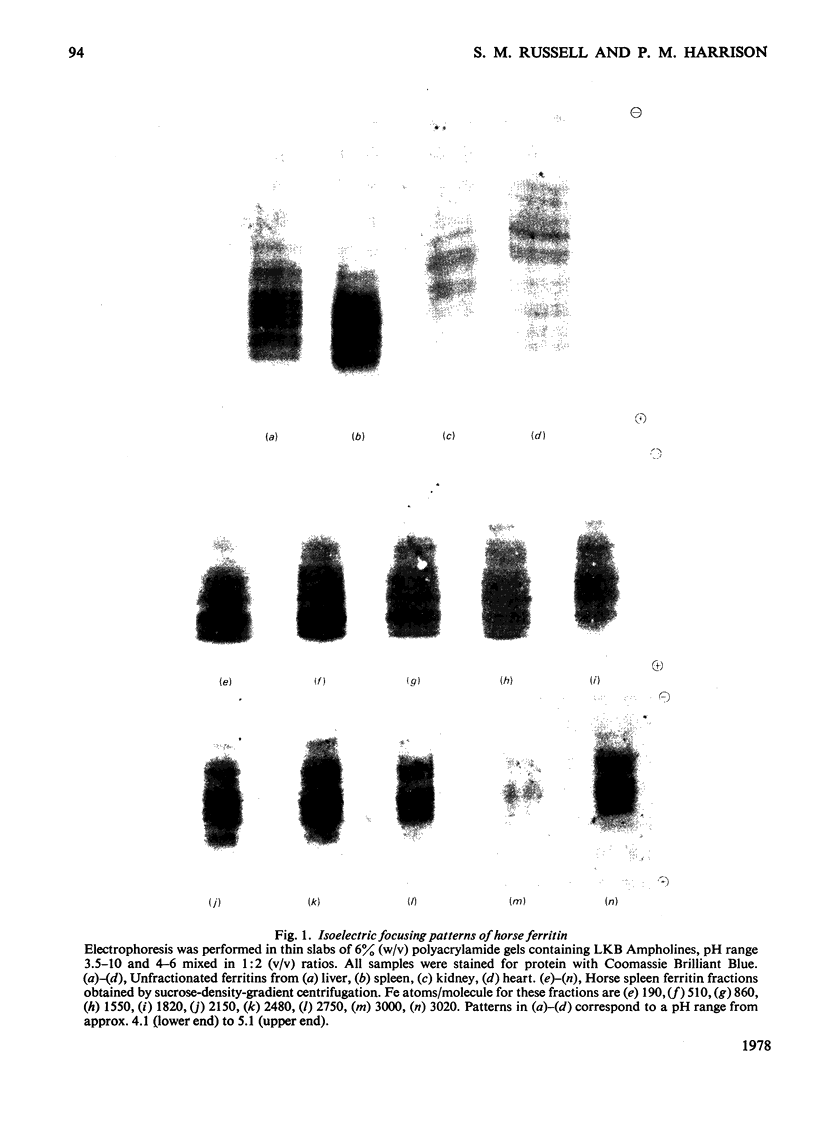
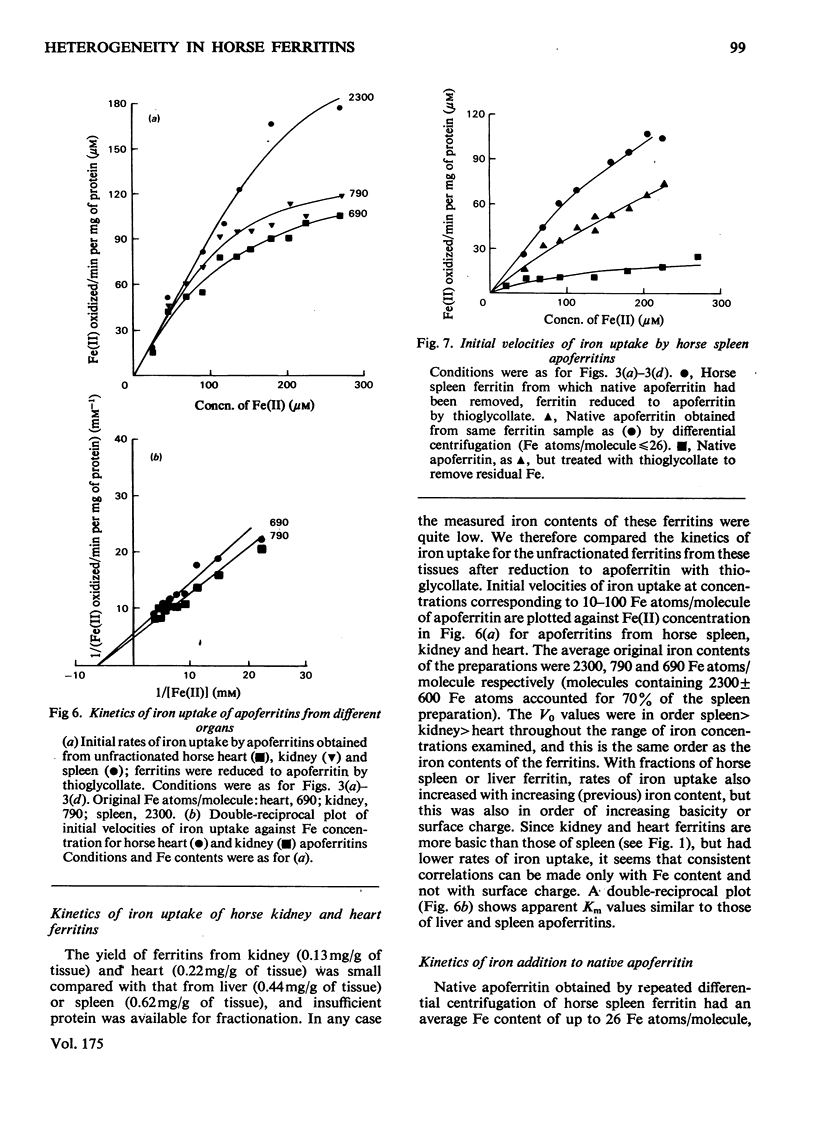
Horse ferritins from different organs show heterogeneity on electrofocusing in Ampholine gradients. Both ferritin and apoferritin from liver and spleen could be fractionated with respect to surface charge by serial precipitation with (NH4)2SO4. In the ferritin fractions, increasing iron content parallels increasing isoelectric point. After removal of their iron, those fractions which originally contained most iron accumulated added iron at the fastest rates. When unfractionated ferritins from different organs were compared the average isoelectric point increased in order spleen less than liver less than kidney less than heart. The order of initial rates of iron uptake by the apoferritins was spleen greater than kidney greater than heart and initial average iron contents also followed this order. The relatively low rates of iron accumulation by iron-poor molecules may have been due to structural alteration, to degradation, to activation of the iron-rich molecules or to other factors.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey C. P., Jr, Lynch E. C., Whitley C. E. Characteristics of ferritin isolated from human marrow, spleen, liver, and reticulocytes. J Lab Clin Med. 1967 Sep;70(3):419–428. [PubMed] [Google Scholar]
- Banyard S. H., Stammers D. K., Harrison P. M. Electron density map of apoferritin at 2.8-A resolution. Nature. 1978 Jan 19;271(5642):282–284. doi: 10.1038/271282a0. [DOI] [PubMed] [Google Scholar]
- Bomford A., Lis Y., McFarlane I. G., Williams R. Variation in the distribution of two human heart ferritin species. Isoferritin profile and subunit composition in normal and iron-overloaded subjects. Biochem J. 1977 Oct 1;167(1):309–312. doi: 10.1042/bj1670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. Microheterogeneity in apoferritin molecules--an artifact. Hoppe Seylers Z Physiol Chem. 1973 Mar;354(3):344–346. [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The catalytic activity of horse spleen apoferritin. Preliminary kinetic studies and the effect of chemical modification. Biochem J. 1973 Jun;133(2):301–309. doi: 10.1042/bj1330301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Millar J. A., Cumming R. L., Bryce C. F. The organ-specificity of ferritin in human and horse liver and spleen. Biochem J. 1973 Jan;131(1):51–59. doi: 10.1042/bj1310051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Adelman T. G., Arosio P., Casareale D., Fitzpatrick P., Harzard J. T., Yokota M. Human isoferritins in normal and disease states. Semin Hematol. 1977 Jan;14(1):71–88. [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Fischbach F. A., Anderegg J. W. An x-ray scattering study of ferritin and apoferritin. J Mol Biol. 1965 Dec;14(2):458–473. doi: 10.1016/s0022-2836(65)80196-6. [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- GIBSON Q. H., MASSEY V., ATHERTON N. M. The nature of compounds present in mixtures of oxidized and reduced flavin mononucleotides. Biochem J. 1962 Nov;85:369–383. doi: 10.1042/bj0850369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda T. G., Gardner F. H. Observations on Fe59 labeled bone marrow ferritin. Blood. 1967 May;29(5):770–779. [PubMed] [Google Scholar]
- Gabuzda T. G., Pearson J. 2 molecular forms of ferritin in rabbit marrow. Nature. 1968 Dec 21;220(5173):1234–1235. doi: 10.1038/2201234a0. [DOI] [PubMed] [Google Scholar]
- HARRISON P. M., HOFMANN T., MAINWARING W. I. The structure of apoferritin: amino acid composition and end-groups. J Mol Biol. 1962 Apr;4:251–256. doi: 10.1016/s0022-2836(62)80003-5. [DOI] [PubMed] [Google Scholar]
- Harrison P. M. Ferritin: an iron-storage molecule. Semin Hematol. 1977 Jan;14(1):55–70. [PubMed] [Google Scholar]
- Harrison P. M., Hoy T. G., Macara I. G., Hoare R. J. Ferritin iron uptake and release. Structure-function relationships. Biochem J. 1974 Nov;143(2):445–451. doi: 10.1042/bj1430445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare R. J., Harrison P. M., Hoy T. G. Structure of horse-spleen apoferritin at 6 angstom resolution. Nature. 1975 Jun 19;255(5510):653–654. doi: 10.1038/255653a0. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Harris H. The investigation of reactive sulphydryls in enzymes and their variants by starch gel electrophoresis. Studies on red cell adenosine deaminase. Ann Hum Genet. 1969 Jul;33(1):81–87. doi: 10.1111/j.1469-1809.1969.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Hoy T. G., Harrison P. M. The uptake of ferric iron by rat liver ferritin in vivo and in vitro. Br J Haematol. 1976 Aug;33(4):497–504. doi: 10.1111/j.1365-2141.1976.tb03568.x. [DOI] [PubMed] [Google Scholar]
- Ishitani K., Listowsky I. Differences in subunit composition and iron content of isoferritins. J Biol Chem. 1975 Jul 25;250(14):5446–5449. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. C., Richter G. W. Distinctive properties of ferritin from the Reuber H-35 rat hepatoma. Cancer Res. 1971 May;31(5):566–572. [PubMed] [Google Scholar]
- Linder-Horowitz M., Ruettinger R. T., Munro H. N. Iron induction of electrophoretically different ferritins in rat liver, heart and kidney. Biochim Biophys Acta. 1970 Mar 31;200(3):442–448. doi: 10.1016/0005-2795(70)90100-5. [DOI] [PubMed] [Google Scholar]
- Linder M. C., Munro H. N. Assay of tissue ferritin. Anal Biochem. 1972 Jul;48(1):266–278. doi: 10.1016/0003-2697(72)90189-3. [DOI] [PubMed] [Google Scholar]
- MAZUR A., LITT I., SHORR E. The relation of sulfhydryl groups in ferritin to its vasodepressor activity. J Biol Chem. 1950 Dec;187(2):485–495. [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Catalytic action of apoferritin. Biochem J. 1973 Oct;135(2):343–348. doi: 10.1042/bj1350343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Kinetics and mechanism of iron uptake. Biochem J. 1972 Jan;126(1):151–162. doi: 10.1042/bj1260151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Drysdale J. W. Role of iron in the regulation of ferritin metabolism. Fed Proc. 1970 Jul-Aug;29(4):1469–1473. [PubMed] [Google Scholar]
- Niederer W. Ferritin: iron incorporation and iron release. Experientia. 1970;26(2):218–220. doi: 10.1007/BF01895596. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Alpert E., Isselbacher K. J., Drysdale J. W. Human isoferritins: organ specific iron and apoferritin distribution. Br J Haematol. 1975 May;30(1):47–55. doi: 10.1111/j.1365-2141.1975.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Powell L. W., McKeering L. V., Halliday J. W. Alterations in tissue ferritins in iron storage disorders. Gut. 1975 Nov;16(11):909–912. doi: 10.1136/gut.16.11.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjyo S., Abe H., Masuda M. Carbohydrate composition of horse spleen ferritin. Biochim Biophys Acta. 1975 Nov 10;411(1):165–167. doi: 10.1016/0304-4165(75)90295-0. [DOI] [PubMed] [Google Scholar]
- Suran A. A., Tarver H. Heterogeneity of horse spleen ferritin and apoferritin: comparison of electrophoretic and chromatographic fractions. Arch Biochem Biophys. 1965 Aug;111(2):399–406. doi: 10.1016/0003-9861(65)90202-x. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Fisher R. A., Harris H. An association between the kinetic and electrophoretic properties of human purine-nucleoside-phosphorylase isozymes. Eur J Biochem. 1971 Dec;24(2):288–295. doi: 10.1111/j.1432-1033.1971.tb19684.x. [DOI] [PubMed] [Google Scholar]
- Urushizaki I., Ishitani K., Niitsu Y. Microheterogeneity of rat liver ferritin: comparison of electrofocusing and chromatographic fractions. Biochim Biophys Acta. 1973 Nov 11;328(1):95–110. doi: 10.1016/0005-2795(73)90334-6. [DOI] [PubMed] [Google Scholar]




