Abstract
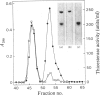
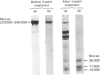
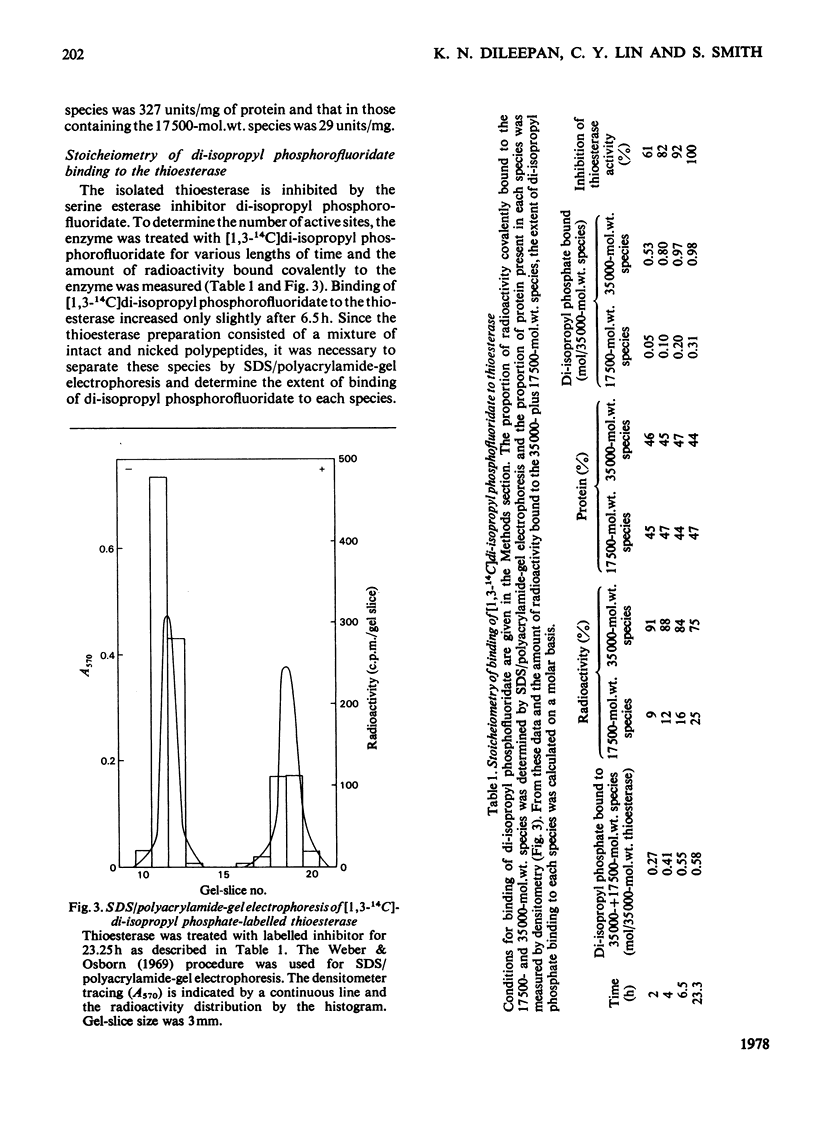
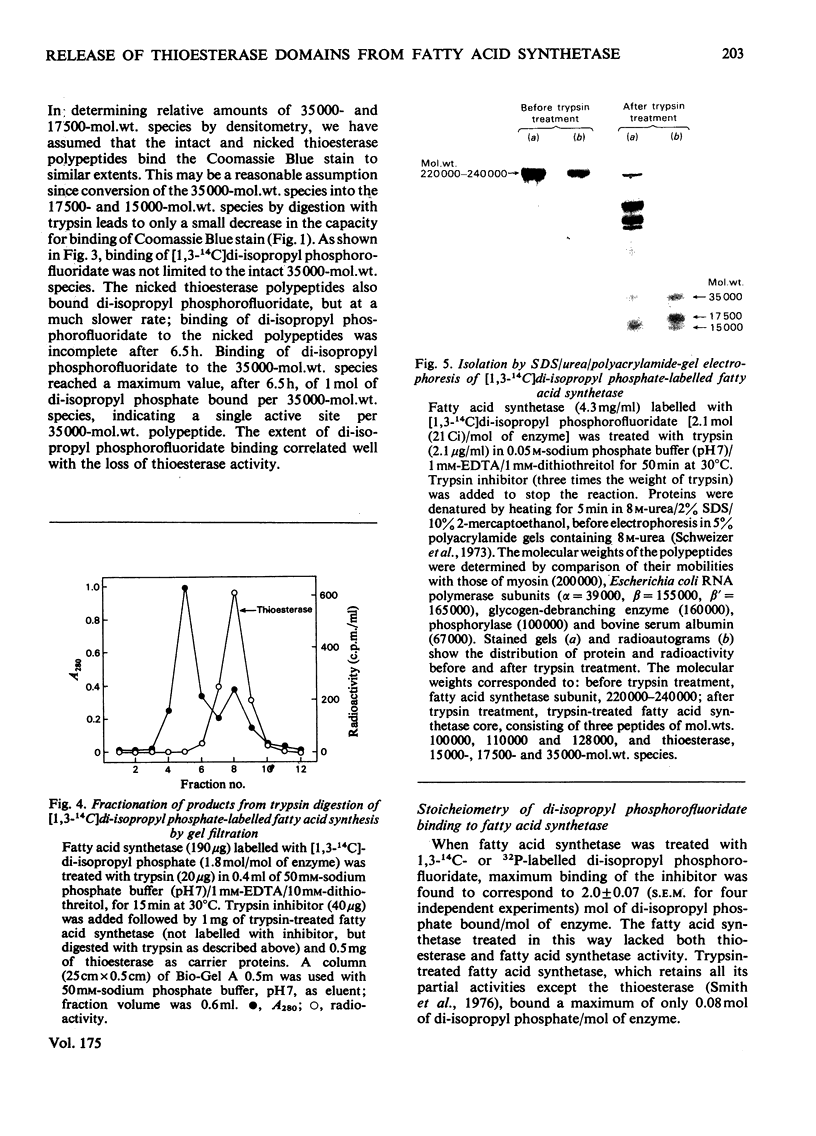
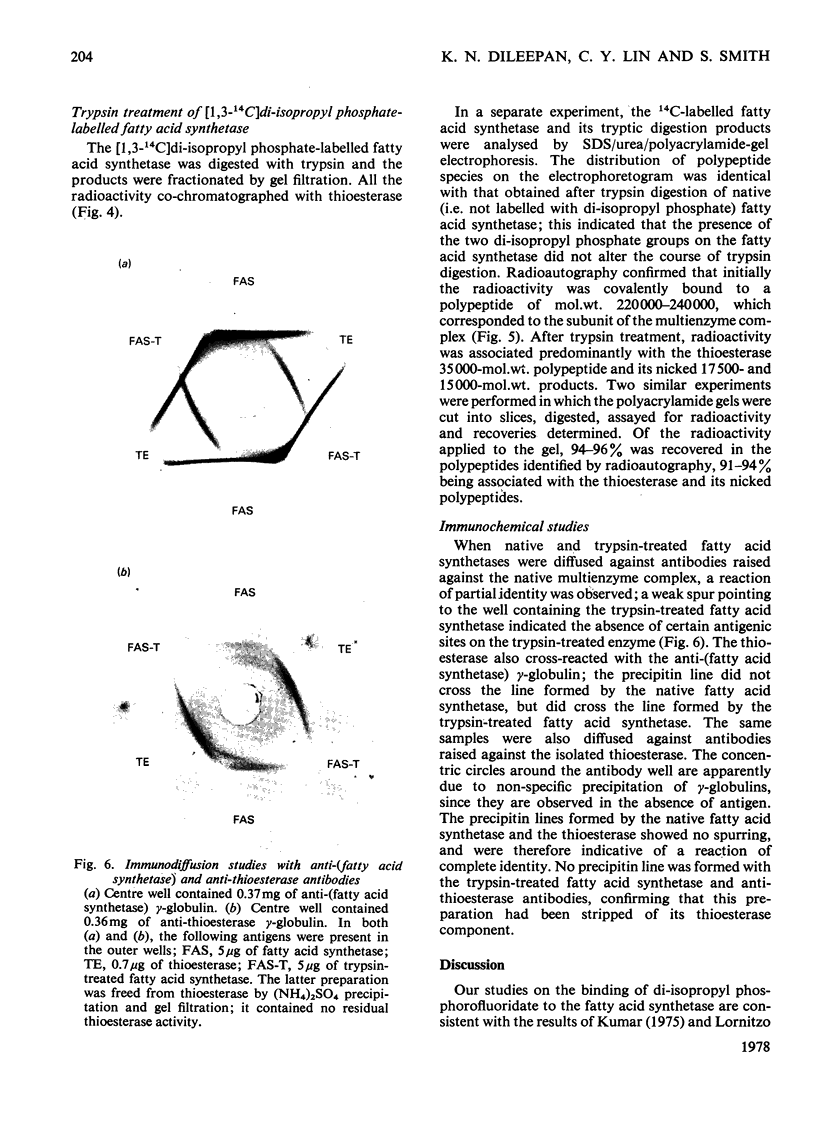
Limited digestion, with trypsin, of the fatty acid synthetase from rat mammary gland releases an enzymically active thioesterase component that, under denaturing conditions, consists of two major species of mol.wts. 35000 and 17500 and a minor species, mol.wt. 15,000. The 17500- and 150000-mol.wt. species are shown to originate from the 35000-mol.wt. species as a result of nicking by trypsin. The nicked polypeptides are enzymically active. The fatty acid synthetase is inhibited by [1,3-14C]di-isopropyl phosphorofluoridate, which is shown to bind to, and inactivate, two thioesterase active sites. When the [1,3-14C]di-isopropyl phosphate-labelled fatty acid synthetase is subjected to limited digestion with trypsin, all of the radioactivity is recovered in the isolated thioesterase component, i.e. in the 35000-mol.wt. polypeptide and its nicked products. Since the isolated thioesterase is shown to bind only one di-isopropyl phosphate residue per 35000-mol.wt. polypeptide, we conclude that the fatty acid synthetase has two thioesterase domains, both of which are removed by limited trypsin treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch K., Vance D. Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem. 1977;46:263–298. doi: 10.1146/annurev.bi.46.070177.001403. [DOI] [PubMed] [Google Scholar]
- Kumar S. Functional deacylases of pigeon liver fatty acid synthetase complex. J Biol Chem. 1975 Jul 10;250(13):5150–5158. [PubMed] [Google Scholar]
- Lin C. Y., Smith S. Properties of the thioesterase component obtained by limited trypsinization of the fatty acid synthetase multienzyme complex. J Biol Chem. 1978 Mar 25;253(6):1954–1962. [PubMed] [Google Scholar]
- Lornitzo F. A., Qureshi A. A., Porter J. W. Subunits of fatty acid synthetase complexes. Enzymatic activities and properties of the half-molecular weight nonidentical subunits of pigeon liver fatty acid synthetase. J Biol Chem. 1975 Jun 25;250(12):4520–4529. [PubMed] [Google Scholar]
- Ringle D. A., Herndon B. L. A micromanipulator-based system for immunologic analyses with microliter and submicroliter reactant volumes. J Immunol. 1965 Nov;95(5):966–979. [PubMed] [Google Scholar]
- Schweizer E., Kniep B., Castorph H., Holzner U. Pantetheine-free mutants of the yeast fatty-acid-synthetase complex. Eur J Biochem. 1973 Nov 15;39(2):353–362. doi: 10.1111/j.1432-1033.1973.tb03133.x. [DOI] [PubMed] [Google Scholar]
- Smith S., Agradi E., Libertini L., Dileepan K. N. Specific release of the thioesterase component of the fatty acid synthetase multienzyme complex by limited trypsinization. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1184–1188. doi: 10.1073/pnas.73.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Studies on the immunological cross-reactivity and physical properties of fatty acid synthetases. Arch Biochem Biophys. 1973 Jun;156(2):751–758. doi: 10.1016/0003-9861(73)90328-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]