Abstract
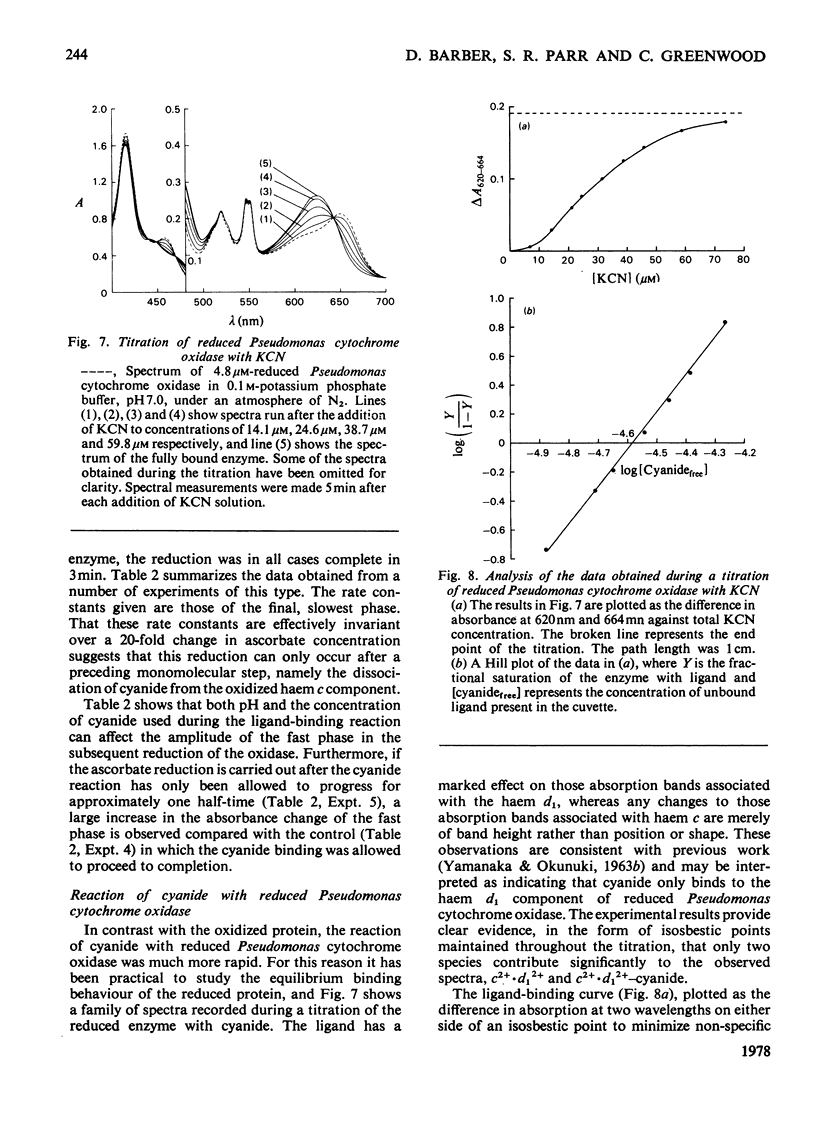
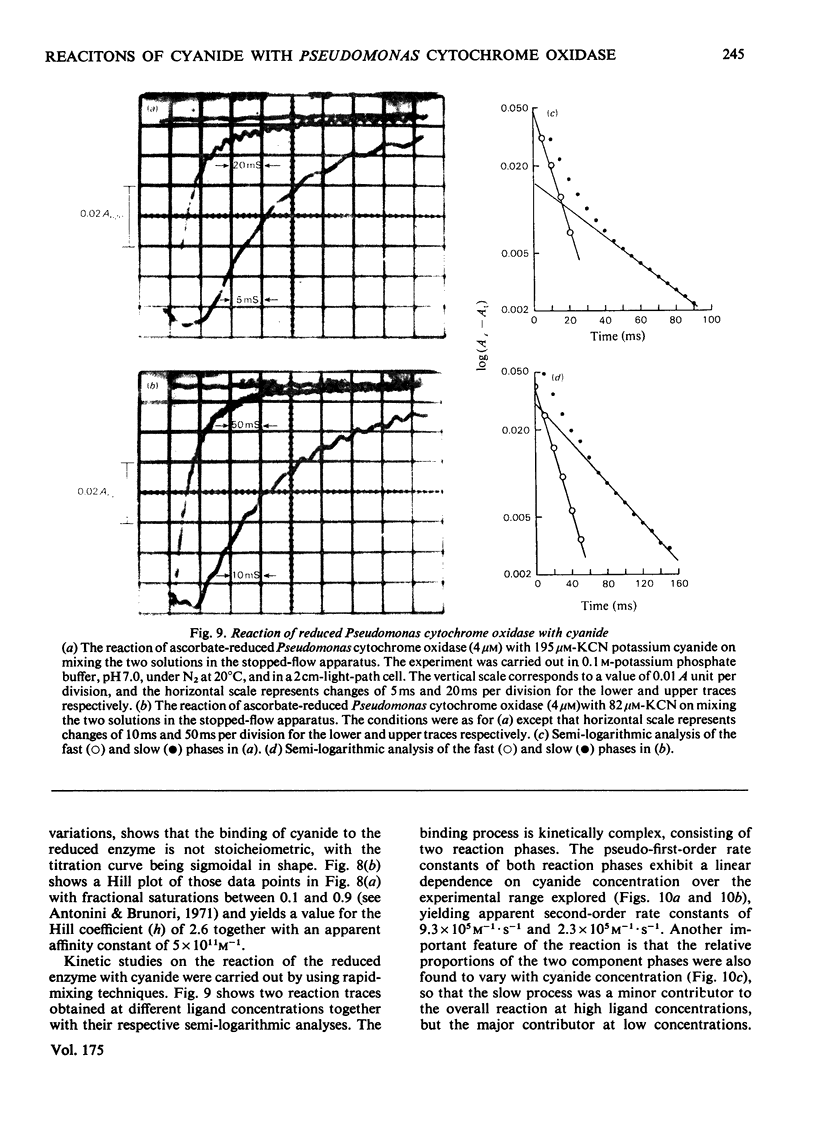
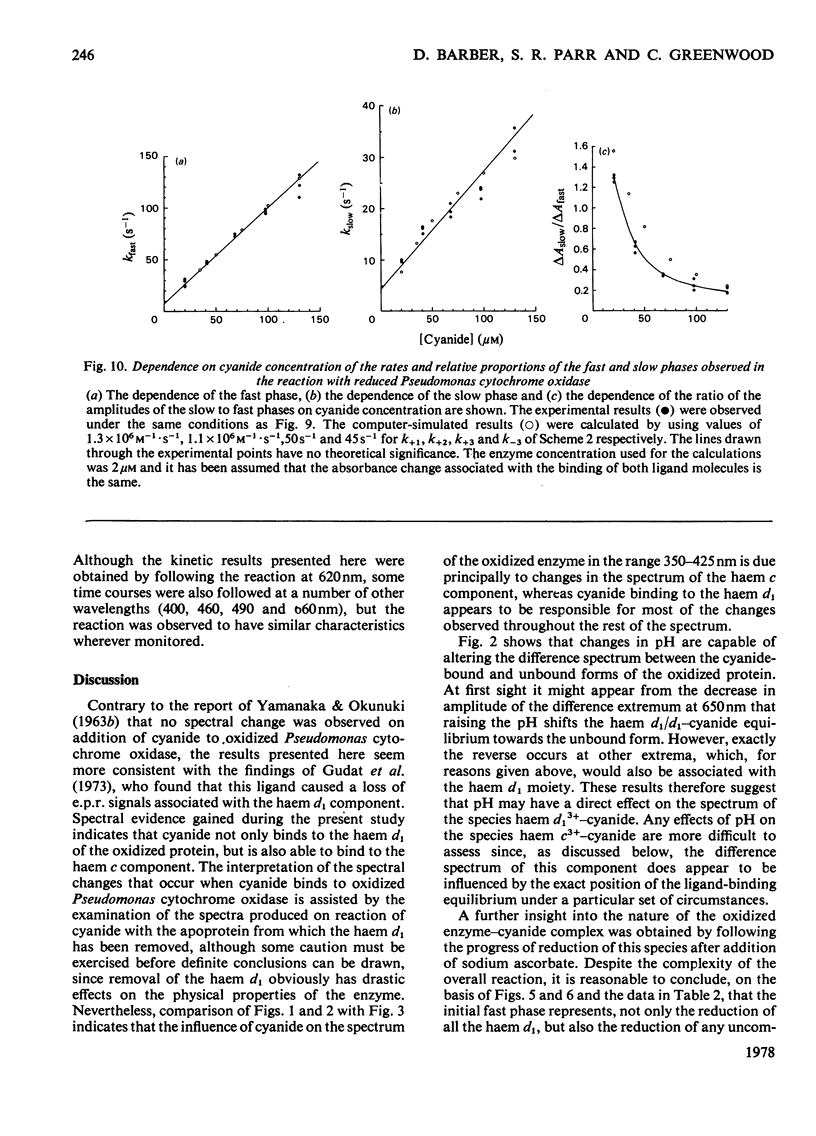
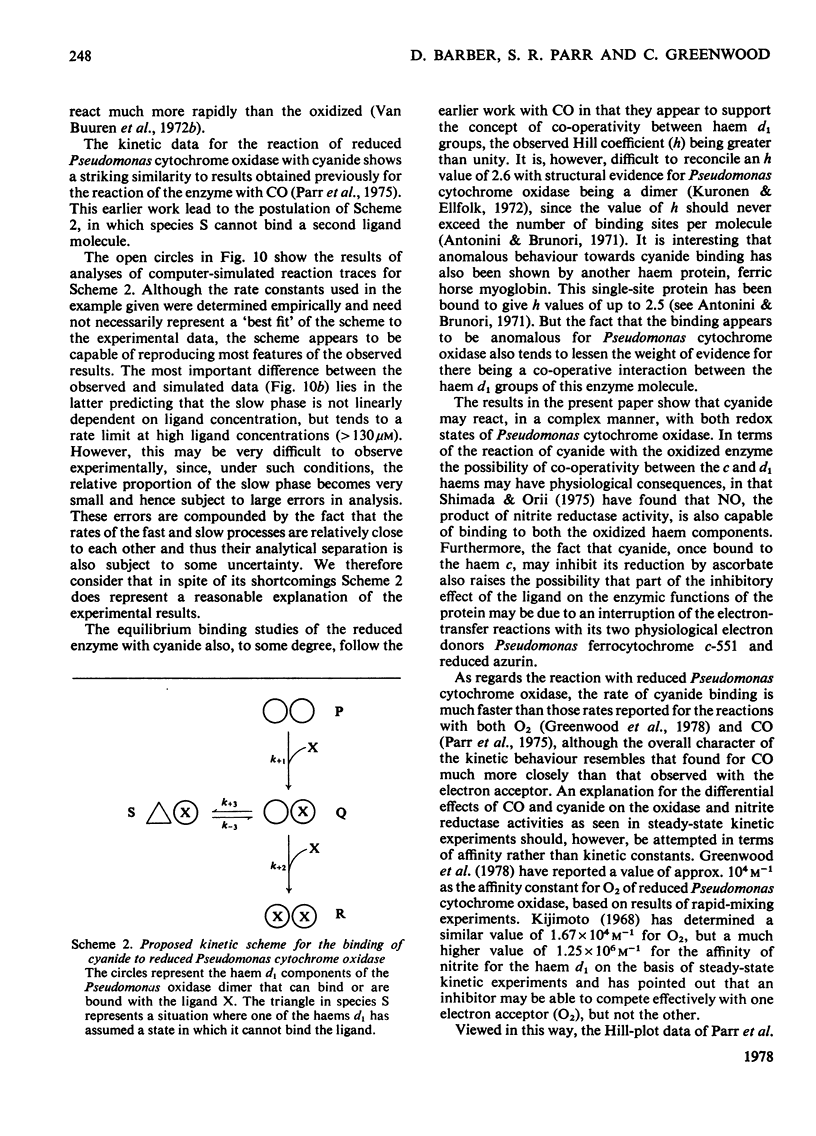
The binding of cyanide to both oxidized and ascorbate-reduced forms of Pseudomonas cytochrome c-551 oxidase was investigated. Spectral studies on the oxidized enzyme and its apoprotein showed that the ligand can bind to both the c and d, haem components of the molecule, and kinetic observations indicated that both chromophores reacted, under a variety of conditions, with very similar rates. Cyanide combination velocities were dependent on ligand concentration, and increasing the pH also accelerated the reaction; the second-order rate constant was estimated as approx. 0.2M-1 . s-1 at pH 7.0. The binding of cyanide to the protein was observed to have a considerable influence on reduction of the enzyme by ascorbate. Spectral and kinetic observations have revealed that the species haem d13+-cyanide and any unbound haem c may react relatively rapidly with the reductant, but the behaviour of cyanide-bound haem c indicates that it may not be reduced without prior dissociation of the ligand, which occurs relatively slowly. The reaction of reduced Pseudomonas cytochrome oxidase with cyanide is radically different from that of the oxidized protein. In this case the ligand only binds to the haem d1 component and reacts much more rapidly. Stopped-flow kinetic measurements showed the binding to be biphasic in form. Both the rates of these processes were dependent on cyanide concentration, with the fast phase having a second-order rate constant of 9.3 X 10(5) M-1 . s-1 and the slow phase one of 2.3 X 10(5) M-1 . s-1. The relative proportions of the two phases also showed a dependency on cyanide concentration, the slower phase increasing as the cyanide concentration decreased. Computer simulations indicate that a reaction scheme originally proposed for the reaction of the enzyme with CO is capable of providing a reasonable explanation of the experimental results. Static-titration data of the reduced enzyme with with cyanide indicated that the binding was non-stoicheiometric, the ligand-binding curve being sigmoidal in shape. A Hill plot of the results yielded a Hill coefficient of 2.6.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D., Parr S. R., Greenwood C. Some spectral and steady-state kinetic properties of Pseudomonas cytochrome oxidase. Biochem J. 1976 Aug 1;157(2):431–438. doi: 10.1042/bj1570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D., Parr S. R., Greenwood C. The reduction of Pseudomonas cytochrome c551 oxidase by chromous ions. Biochem J. 1977 Jun 1;163(3):629–632. doi: 10.1042/bj1630629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE P., TSOU C. L. Reaction between hydrocyanic acid, cyanide ion and ferricytochrome c. Biochem J. 1952 Feb;50(4):440–448. doi: 10.1042/bj0500440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Barber D., Parr S. R., Antonini E., Brunori M., Colosimo A. The reaction of Pseudomonas aeruginosa cytochrome c-551 oxidase with oxygen. Biochem J. 1978 Jul 1;173(1):11–17. doi: 10.1042/bj1730011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudat J. C., Singh J., Wharton D. C. Cytochrome oxidase from Pseudomonas aeruginosa. I. Purification and some properties. Biochim Biophys Acta. 1973 Feb 22;292(2):376–390. doi: 10.1016/0005-2728(73)90044-3. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Kuronen T., Ellfolk N. A new purification procedure and molecular properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1972 Sep 20;275(3):308–318. doi: 10.1016/0005-2728(72)90212-5. [DOI] [PubMed] [Google Scholar]
- Kuronen T., Saraste M., Ellfork N. The subunit structure of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1975 May 30;393(1):48–54. doi: 10.1016/0005-2795(75)90215-9. [DOI] [PubMed] [Google Scholar]
- Orii Y., Shimada H., Nozawa T., Hatano M. The interaction between the heme c and heme d moieties of Pseudomonas nitrite reductase as revealed by magnetic and natural circular dichroism studies. Biochem Biophys Res Commun. 1977 Jun 20;76(4):983–988. doi: 10.1016/0006-291x(77)90952-4. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C., Brunori M. The electron-transfer reaction between azurin and the cytochrome c oxidase from Pseudomonas aeruginosa. Biochem J. 1977 Nov 1;167(2):447–455. doi: 10.1042/bj1670447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeruginosa cytochrome c oxidase with carbon monoxide. Biochem J. 1975 Oct;151(1):51–59. doi: 10.1042/bj1510051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Virtanen I., Kuronen T. The quatenary structure of Pseudomonas cytochrome oxidase studied by electron microscopy. Biochim Biophys Acta. 1977 May 27;492(1):156–162. doi: 10.1016/0005-2795(77)90222-7. [DOI] [PubMed] [Google Scholar]
- Shimada H., Orii Y. The nitric oxide compounds of Pseudomonas aeruginosa nitrite reductase and their probable participation in the nitrite reduction. FEBS Lett. 1975 Jun 15;54(2):237–240. doi: 10.1016/0014-5793(75)80082-2. [DOI] [PubMed] [Google Scholar]
- Wharton D. C., Gudat J. C., Gibson Q. H. Cytochrome oxidase from Pseudomonas aeruginosa. I. Reaction with copper protein. Biochim Biophys Acta. 1973 Apr 5;292(3):611–620. doi: 10.1016/0005-2728(73)90009-1. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., KIJIMOTO S., OKUNUKI K. Biological significance of Pseudomonas cytochrome oxidase in Pseudomonas aeruginosa. J Biochem. 1963 May;53:416–421. doi: 10.1093/oxfordjournals.jbchem.a127716. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. Crystalline Pseudomonas cytochrome oxidase. I. Enzymic properties with special reference to the biological specificity. Biochim Biophys Acta. 1963 Mar 12;67:379–393. doi: 10.1016/0006-3002(63)91844-4. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. Crystalline Pseudomonas cytochrome oxidase. II. Spectral properties of the enzyme. Biochim Biophys Acta. 1963 Mar 12;67:394–406. doi: 10.1016/0006-3002(63)91845-6. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. RECONSTITUTION OF PSEUDOMONAS CYTOCHROME OXIDASE FROM HAEM A2 AND ITS PROTEIN MOIETY AND SOME PROPERTIES OF THE RECONSTITUTED ENZYME. Biochem Z. 1963;338:62–72. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- van Buuren K. J., Zuurendonk P. F., van Gelder B. F., Muijsers A. O. Biochemical and biophysical studies on cytochrome aa 3 . V. Binding of cyanide to cytochrome aa 3 . Biochim Biophys Acta. 1972 Feb 28;256(2):243–257. doi: 10.1016/0005-2728(72)90056-4. [DOI] [PubMed] [Google Scholar]