Abstract
beta-Bungarotoxin was labelled with pyridoxal 5'-phosphate (incorporating 3H). The kinetics of beta-bungarotoxin binding to several tissue subfragments of nervous tissue was studied. The dissociation constant of 3H-pyridoxylated beta-bungarotoxin in this reaction was 0.21-0.37 micron and that of unlabelled beta-bungarotoxin was 25 nM. Hill [(1910) J. Physiol. (London) 40, iv-vii] and Scatchard [(1949) Ann. N.Y. Acad. Sci. 51, 660-672] analyses demonstrated no co-operativity of binding and only a single class of receptor sites, consistent with a bimolecular association of beta-bungarotoxin and its receptor. The iodinated toxin was physiologically inactive. Toxin was bound in non-specific unsaturable fashion by glass and/or plastic. This low-affinity binding was corrected by addition of bovine serum albumin to a final concentration of 30 mg/ml. A soluble protein receptor of beta-bungarotoxin was isolated and the mol.wt. is approx. 200000.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
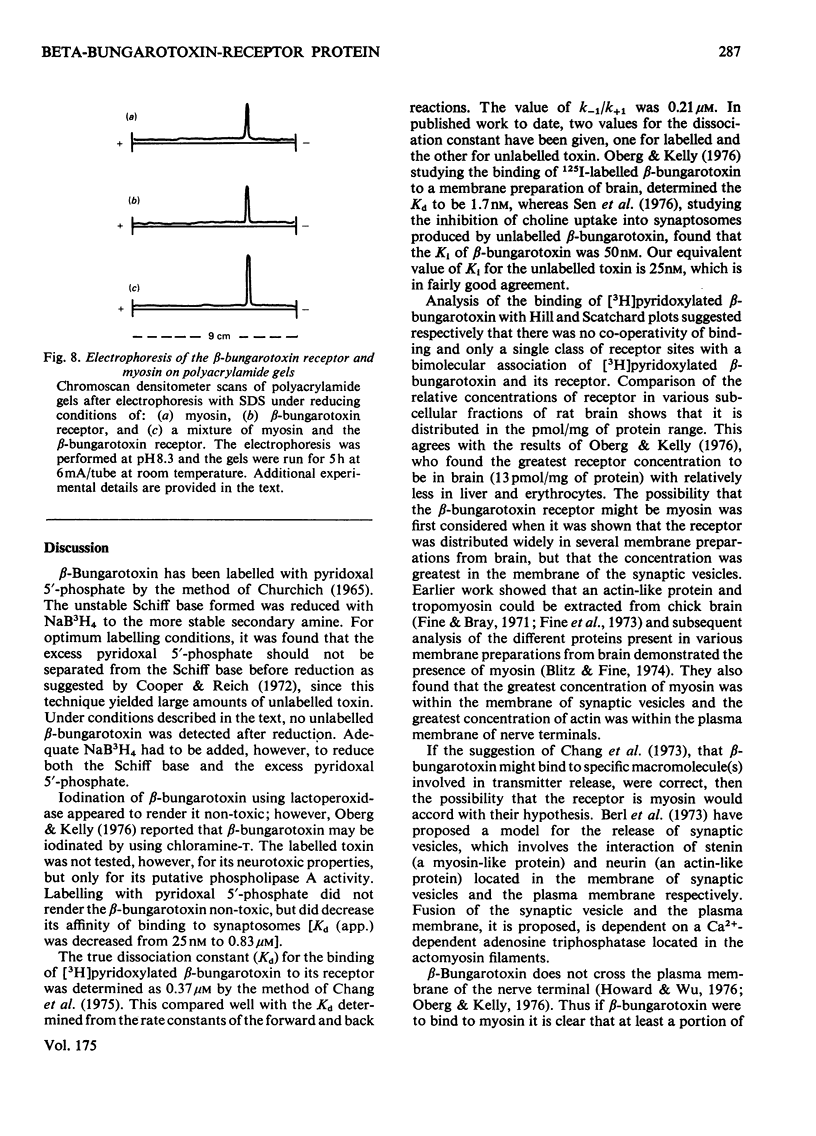
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Blitz A. L., Fine R. E. Muscle-like contractile proteins and tubulin in synaptosomes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4472–4476. doi: 10.1073/pnas.71.11.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Bray D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J Mol Biol. 1975 Nov 25;99(1):1–14. doi: 10.1016/s0022-2836(75)80154-9. [DOI] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Chang C. C., Chen T. F., Lee C. Y. Studies of the presynaptic effect of -bungarotoxin on neuromuscular transmission. J Pharmacol Exp Ther. 1973 Feb;184(2):339–345. [PubMed] [Google Scholar]
- Chang K. J., Jacobs S., Cuatrecasas P. Quantitative aspects of hormone-receptor interactions of high affinity. Effect of receptor concentration and measurement of dissociation constants of labeled and unlabeled hormones. Biochim Biophys Acta. 1975 Oct 6;406(2):294–303. doi: 10.1016/0005-2736(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Churchich J. E. Energy transfer in protein pyridoxamine-5-phosphate conjugates. Biochemistry. 1965 Jul;4(7):1405–1410. doi: 10.1021/bi00883a027. [DOI] [PubMed] [Google Scholar]
- Cooper D., Reich E. Neurotoxin from venom of the cobra, Naja naja siamensis. Purification and radioactive labeling. J Biol Chem. 1972 May 25;247(10):3008–3013. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L., Hitchcock S. E., Kaminer B. Tropomyosin in brain and growing neurones. Nat New Biol. 1973 Oct 10;245(145):182–186. doi: 10.1038/newbio245182a0. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Bray D. Actin in growing nerve cells. Nat New Biol. 1971 Nov 24;234(47):115–118. doi: 10.1038/newbio234115a0. [DOI] [PubMed] [Google Scholar]
- Howard B. D. Effects of beta-bungarotoxin on mitochondrial respiration are caused by associated phospholipase A activity. Biochem Biophys Res Commun. 1975 Nov 3;67(1):58–65. doi: 10.1016/0006-291x(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Howard B. D., Wu W. C. Evidence that beta-bungarotoxin acts at the exterior of nerve terminals. Brain Res. 1976 Feb 13;103(1):190–192. doi: 10.1016/0006-8993(76)90704-6. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Oberg S. G., Strong P. N., Wagner G. M. beta-Bungarotoxin, a phospholipase that stimulates transmitter release. Cold Spring Harb Symp Quant Biol. 1976;40:117–125. doi: 10.1101/sqb.1976.040.01.013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDermot J., Westgaard R. H., Thompson E. J. beta-Bungarotoxin. Separation of two discrete proteins with different synaptic actions. Biochem J. 1978 Oct 1;175(1):271–279. doi: 10.1042/bj1750271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg S. G., Kelly R. B. Saturable binding to cell membranes of the presynaptic neurotoxin, beta-bungarotoxin. Biochim Biophys Acta. 1976 May 21;433(3):662–673. doi: 10.1016/0005-2736(76)90289-3. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Sen I., Grantham P. A., Cooper J. R. Mechanism of action of beta-bungarotoxin on synaptosomal preparations. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2664–2668. doi: 10.1073/pnas.73.8.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong P. N., Goerke J., Oberg S. G., Kelly R. B. beta-Bungarotoxin, a pre-synaptic toxin with enzymatic activity. Proc Natl Acad Sci U S A. 1976 Jan;73(1):178–182. doi: 10.1073/pnas.73.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wernicke J. F., Vanker A. D., Howard B. D. The mechanism of action of beta-bungarotoxin. J Neurochem. 1975 Oct;25(4):483–496. doi: 10.1111/j.1471-4159.1975.tb04354.x. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Ostlund R. E., Pastan I. Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4144–4148. doi: 10.1073/pnas.71.10.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]


