Abstract
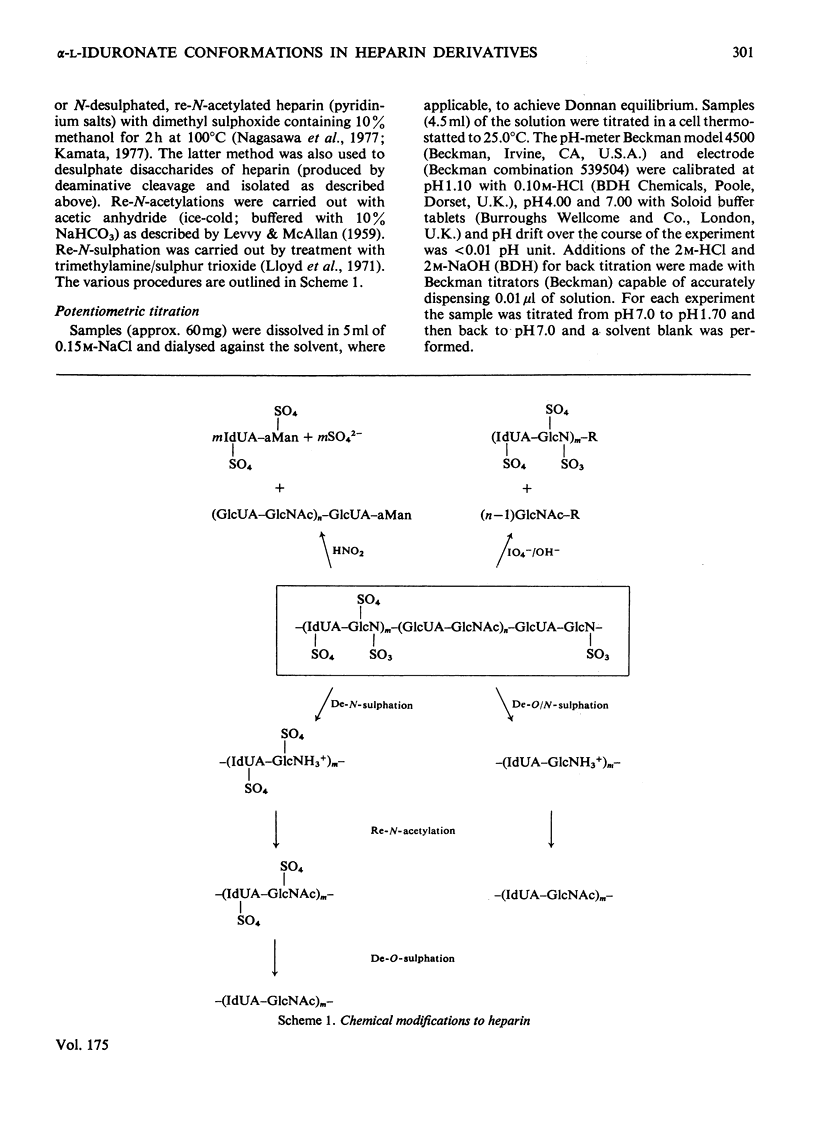
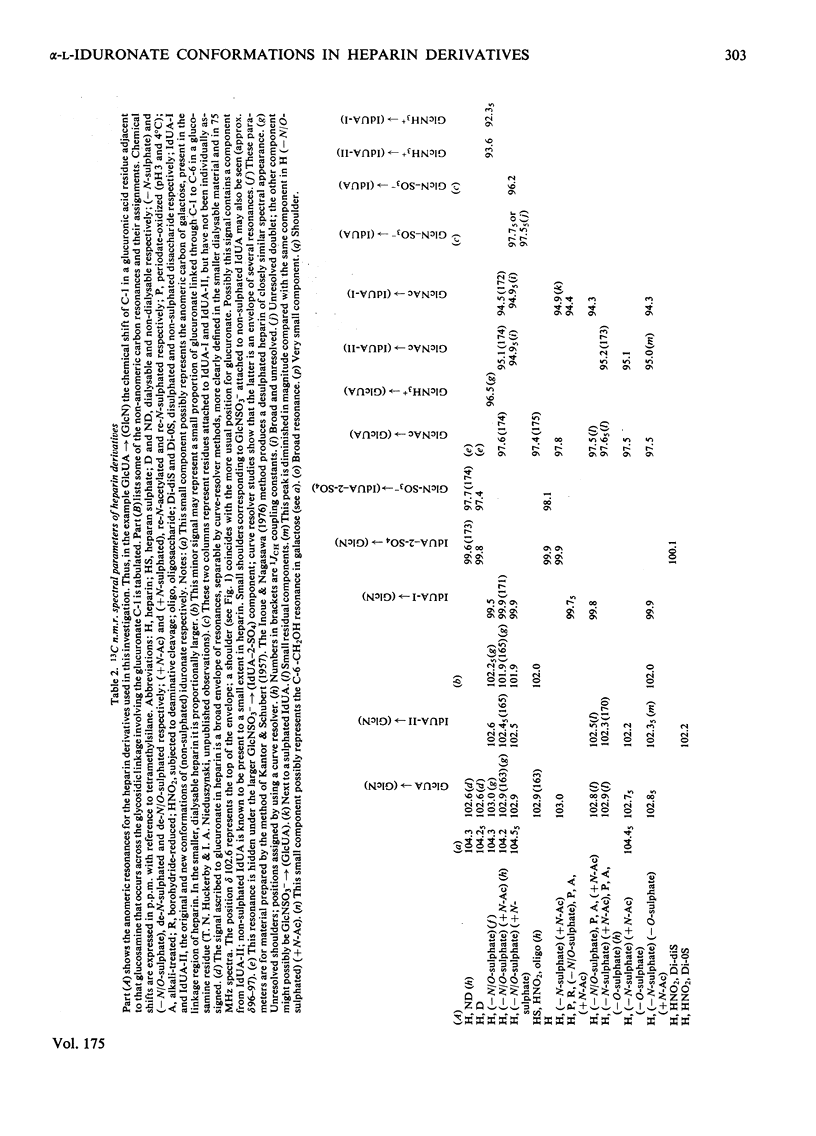
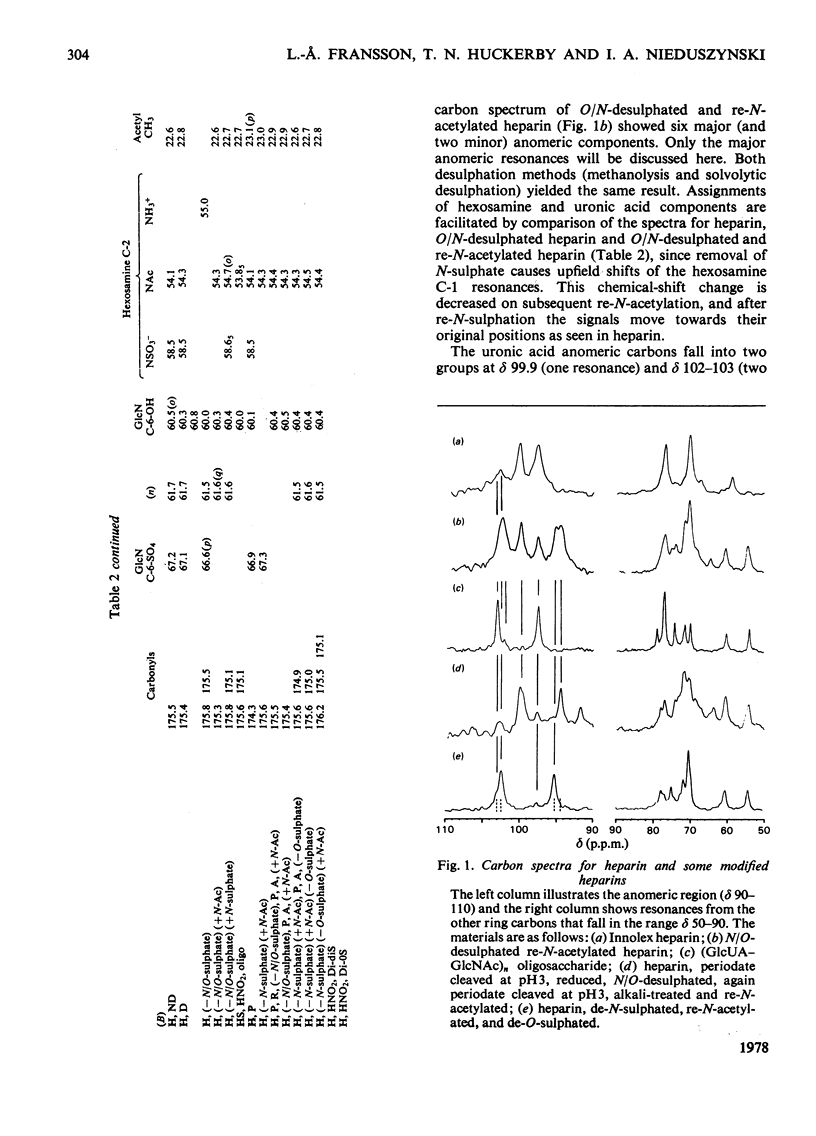
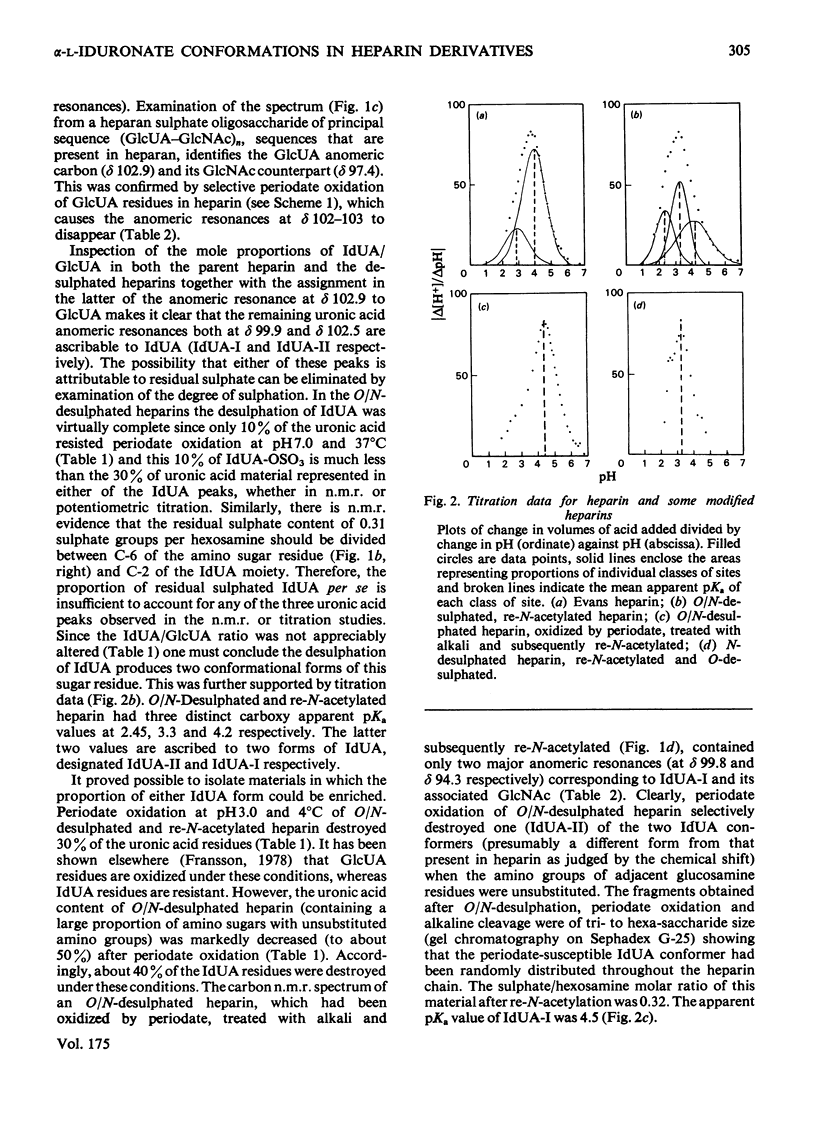
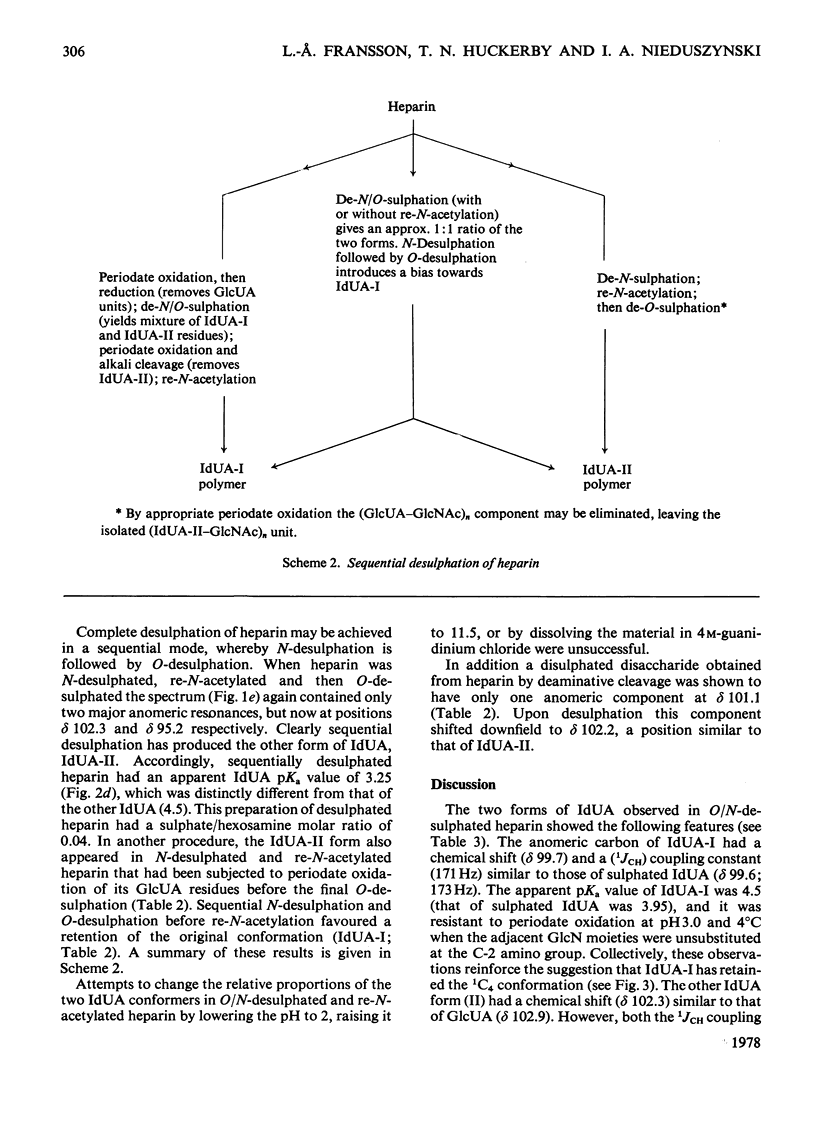
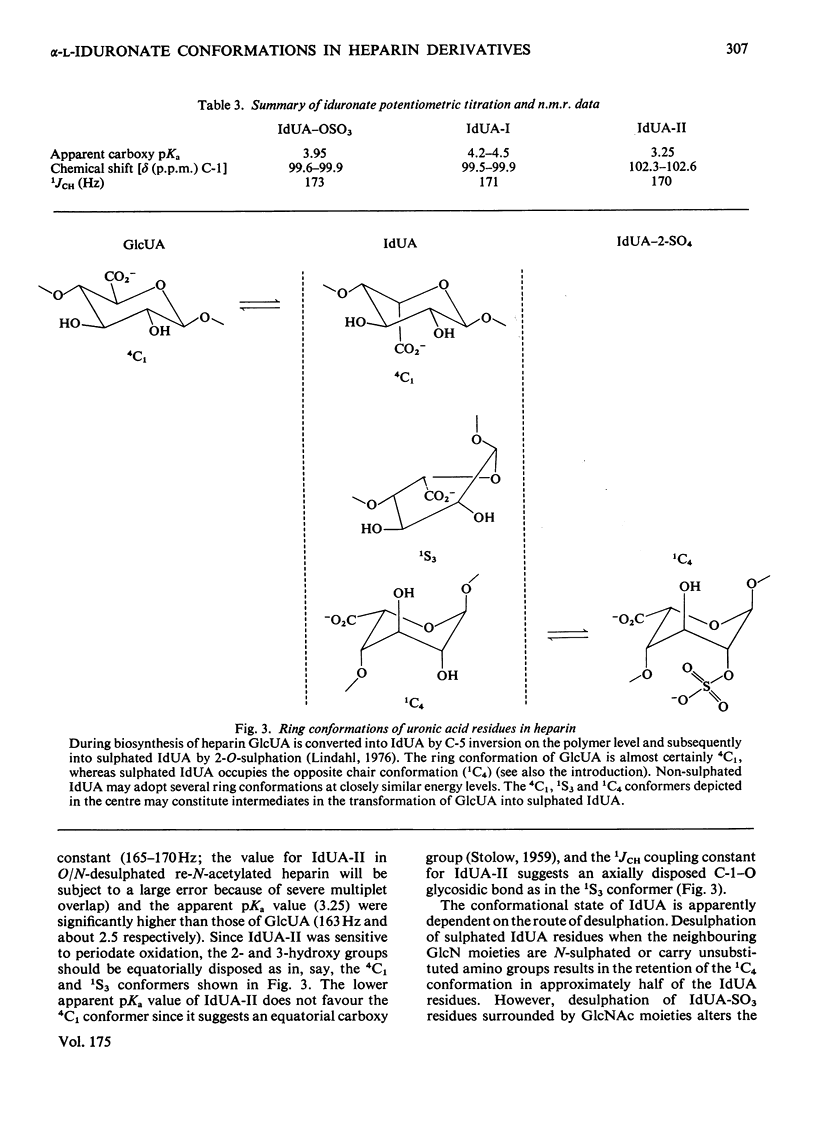
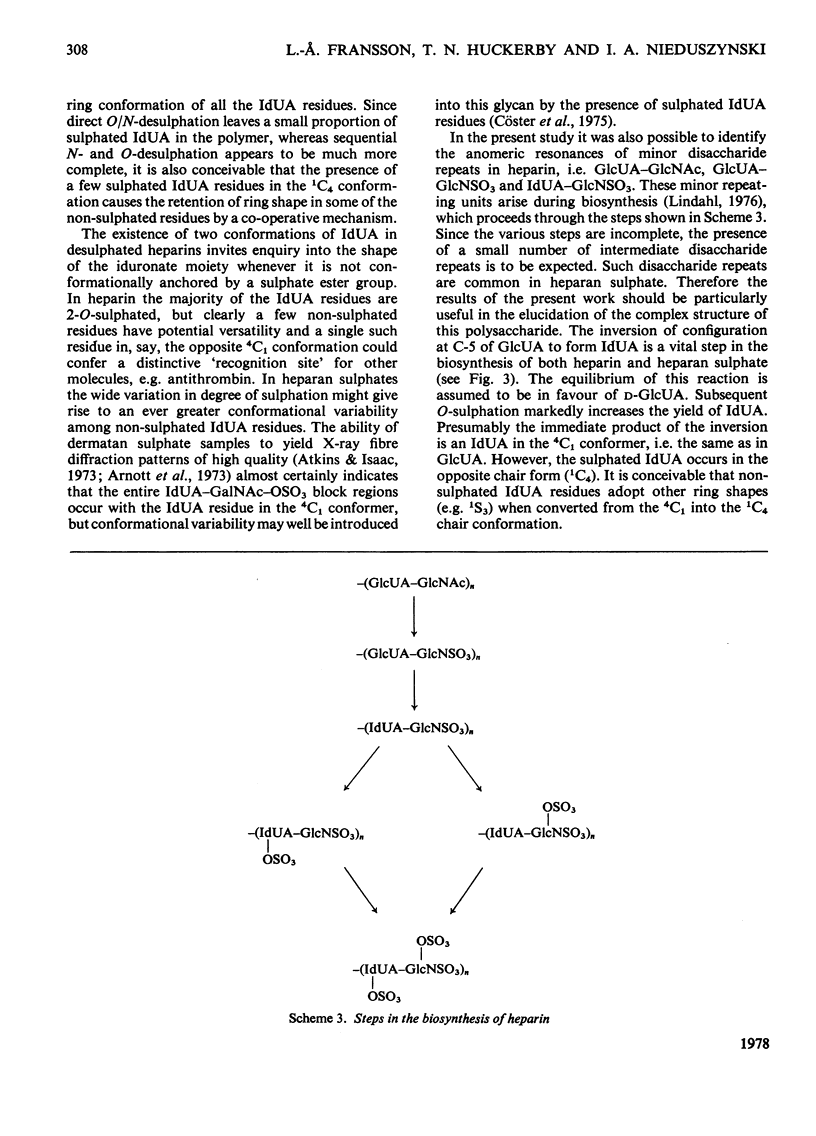
A heparin derivative that had been O/N-desulphated and re-N-acetylated was investigated by 13C n.m.r. spectroscopy and potentiometric titration. Three forms of uronic acid were observed, tentatively identified as beta-D-glucuronate, and two different forms of alpha-L-iduronate. A comparison of the n.m.r. spectra of heparin, an oligosaccharide (beta-D-glucuronate-2-acetamido-2-deoxy-alpha-D-glucose)n, and heparin that had been subjected to selective oxidation of beta-D-glucuronate, enabled the position of the anomeric carbon of the latter residue to be assigned [delta 102.9 (p.p.m.)]. Periodate oxidation of O/N-desulphated heparin destroyed in addition, approx. 40% of the alpha-L-iduronate content. The remainder of the alpha-L-iduronate residues displayed only one anomeric resonance, at delta 99.7 (p.p.m.). In another preparation, after sequential desulphation of heparin (N-desulphation, re-N-acetylation and O-desulphation) the anomeric resonance of the alpha-L-iduronate residue shifted downfield [from delta99.7 (p.p.m.) to delta 102.3]indicating a change in ring conformation. These data support the interpretation that the unsulphated alpha-L-iduronate residues may adopt two conformations. It was shown that the proportions of alpha-L-iduronate conformers are determined by the sequence of desulphation operations. Also minor components of heparin were assigned.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Guss J. M., Hukins D. W., Matthews M. B. Dermatan sulfate and chondroitin 6-sulfate conformations. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1377–1383. doi: 10.1016/0006-291x(73)91139-x. [DOI] [PubMed] [Google Scholar]
- Atkins E. D., Isaac D. H. X-ray diffraction studies on the connective tissue polysaccharides. Molecular conformations of dermatan sulphate. J Mol Biol. 1973 Nov 15;80(4):773–779. doi: 10.1016/0022-2836(73)90209-x. [DOI] [PubMed] [Google Scholar]
- Atkins E. D., Nieduszynski I. A. Effect of alpha-L-iduronate conformation on the molecular shape of heparin. Fed Proc. 1977 Jan;36(1):78–83. [PubMed] [Google Scholar]
- Cöster L., Malmström A., Sjöberg I., Fransson L. The co-polymeric structure of pig skin dermatan sulphate. Distribution of L-iduronic acid sulphate residues in co-polymeric chains. Biochem J. 1975 Feb;145(2):379–389. doi: 10.1042/bj1450379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- Elloway H. F., Atkins E. D. Molecular conformation of sodium heparan sulphate in the condensed phase. Biochem J. 1977 Mar 1;161(3):495–498. doi: 10.1042/bj1610495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A. Interaction between dermatan sulphate chains. I. Affinity chromatography of copolymeric galactosaminioglycans on dermatan sulphate-substituted agarose. Biochim Biophys Acta. 1976 Jun 23;437(1):106–115. doi: 10.1016/0304-4165(76)90351-2. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Periodate oxidation of L-iduronic acid residues in dermatan sulphate. Carbohydr Res. 1974 Sep;36(2):339–348. doi: 10.1016/s0008-6215(00)83055-4. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Rodén L., Spach M. L. Automated ion-exchange chromatography of uronic acids and uronic acid containing oligosaccharides. Anal Biochem. 1968 May;23(2):317–330. doi: 10.1016/0003-2697(68)90362-x. [DOI] [PubMed] [Google Scholar]
- Hamer G. K., Perlin A. S. A 13C-N.M.R. spectral study of chondroitin sulfates A, B, and C: evidence of heterogeneity. Carbohydr Res. 1976 Jul;49:37–48. doi: 10.1016/s0008-6215(00)83123-7. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976 Jan;46(1):87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Kraemer P. M. Heparin releases heparan sulfate from the cell surface. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1334–1340. doi: 10.1016/0006-291x(77)91438-3. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. G., Embery G., Fowler L. J. Studies on heparin degradation. I. Preparation of ( 35 S) sulphamate derivatives for studies on heparin degrading enzymes of mammalian origin. Biochem Pharmacol. 1971 Mar;20(3):637–648. doi: 10.1016/0006-2952(71)90150-x. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Inoue Y., Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977 Sep;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Inoue Y., Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977 Sep;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- Nieduszynski I. A., Atkins E. D. Conformation of the mucopolysaccharides. X-ray fibre diffraction of heparin. Biochem J. 1973 Dec;135(4):729–733. doi: 10.1042/bj1350729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Scott J. E. Perodate oxidation, pKa and conformation of hexuronic acids in polyuronides and mucopolysaccharides. Biochim Biophys Acta. 1968 Dec 23;170(2):471–473. doi: 10.1016/0304-4165(68)90040-8. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]


