Abstract
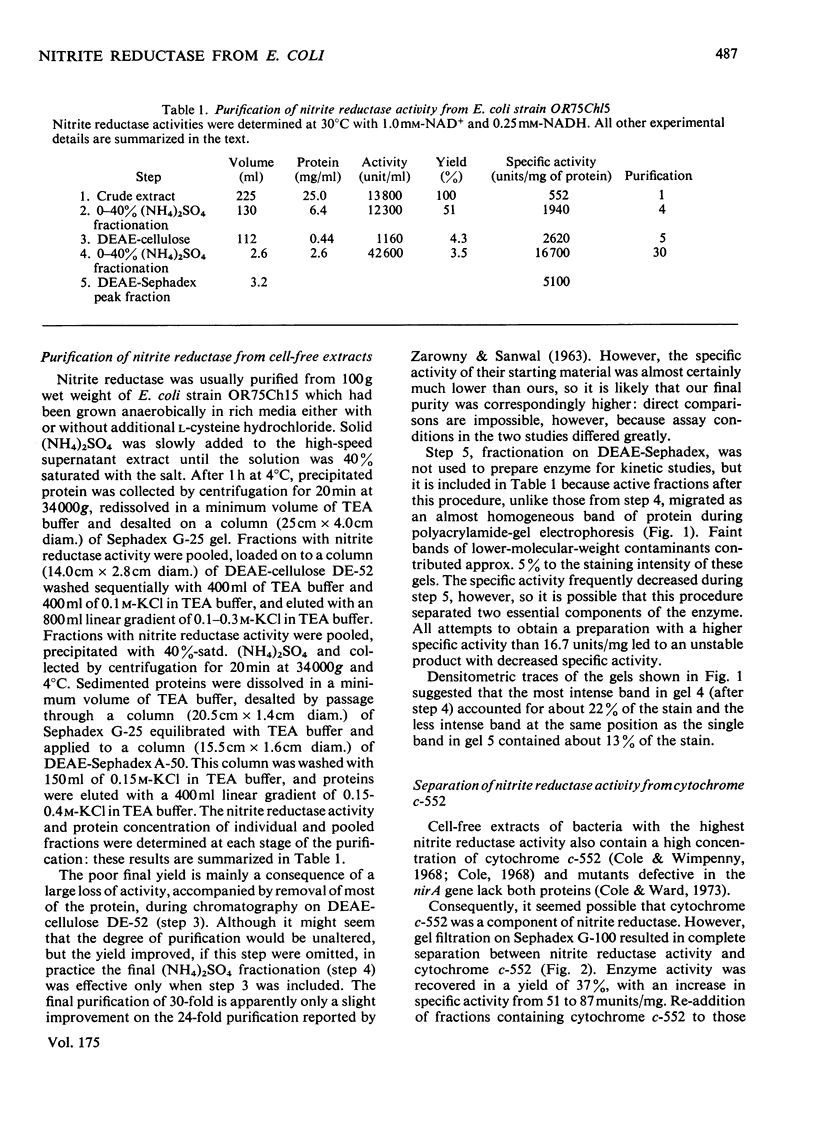
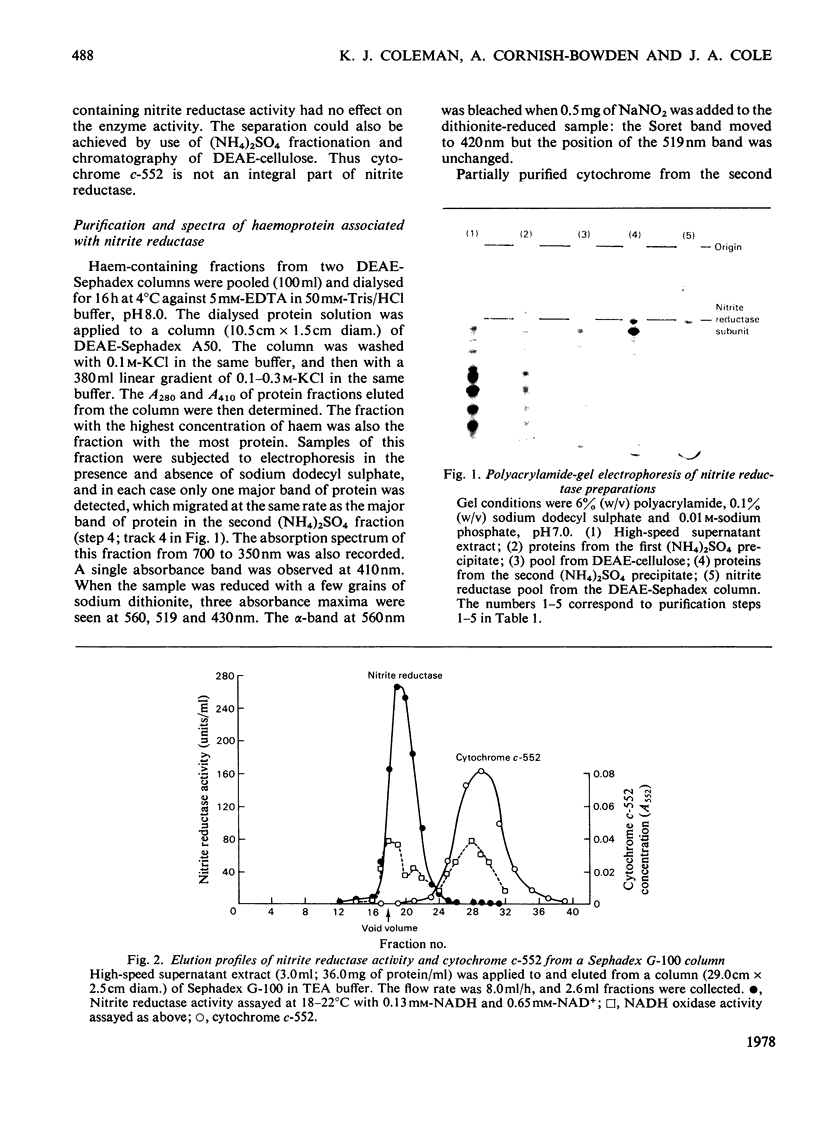
NADH-nitrite oxidoreductase (EC 1.6.4) was purified to better than 95% homogeneity from batch cultures of Escherichia coli strain OR75Ch15, which is partially constitutive for nitrite reductase synthesis. Yields of purified enzyme were low, mainly because of a large loss of activity during chromatography on DEAE-cellulose. The quantitative separation of cytochrome c-552 from nitrite reductase activity resulted in an increase in the specific activity of the enzyme: this cytochrome is not therefore an integral part of nitrite reductase. The subunit molecular weights of nitrite reductase and of a haemoprotein contaminant, as determined by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, were 88000 and 80000 respectively. The sedimentation coefficient was calculated to be in the range 8.5-9.5S, consistent with a mol.wt. of 190000. It is suggested therefore that the native enzyme is a dimer with two identical or similar-sized subunits. Purest samples contained 0.4 mol of flavin/mol of enzyme, but no detectable haem. Catalytic activity was totally inhibited by 20 micron-p-chloromercuribenzoate and 1 mM-cyanide, slightly inhibited by 1 micron-sulphite and 10mM-arsenite, but insensitive to 1 mM-2,2'-bipyridine, 4mM-1,10-phenanthroline and 10mM-NaN3. Three molecules of NADH were oxidized for each NO2-ion reduced: the product of the reaction is therefore assumed to be NH4+. The specific activity of hydroxylamine reductase increased at each step in the purification of nitrite reductase, and the elution profiles for these two activities during chromatography on DEAE-Sephadex were coincident. It is likely that a single enzyme is responsible for both activities.
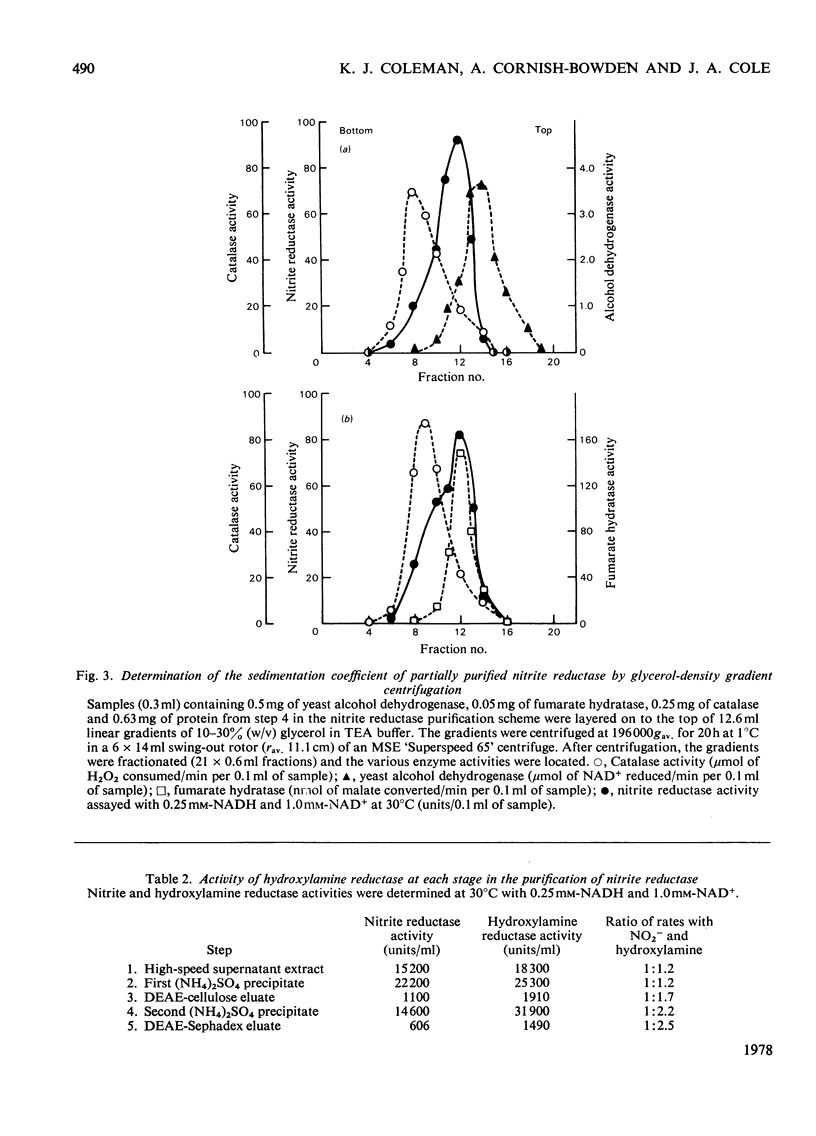
Full text
PDF




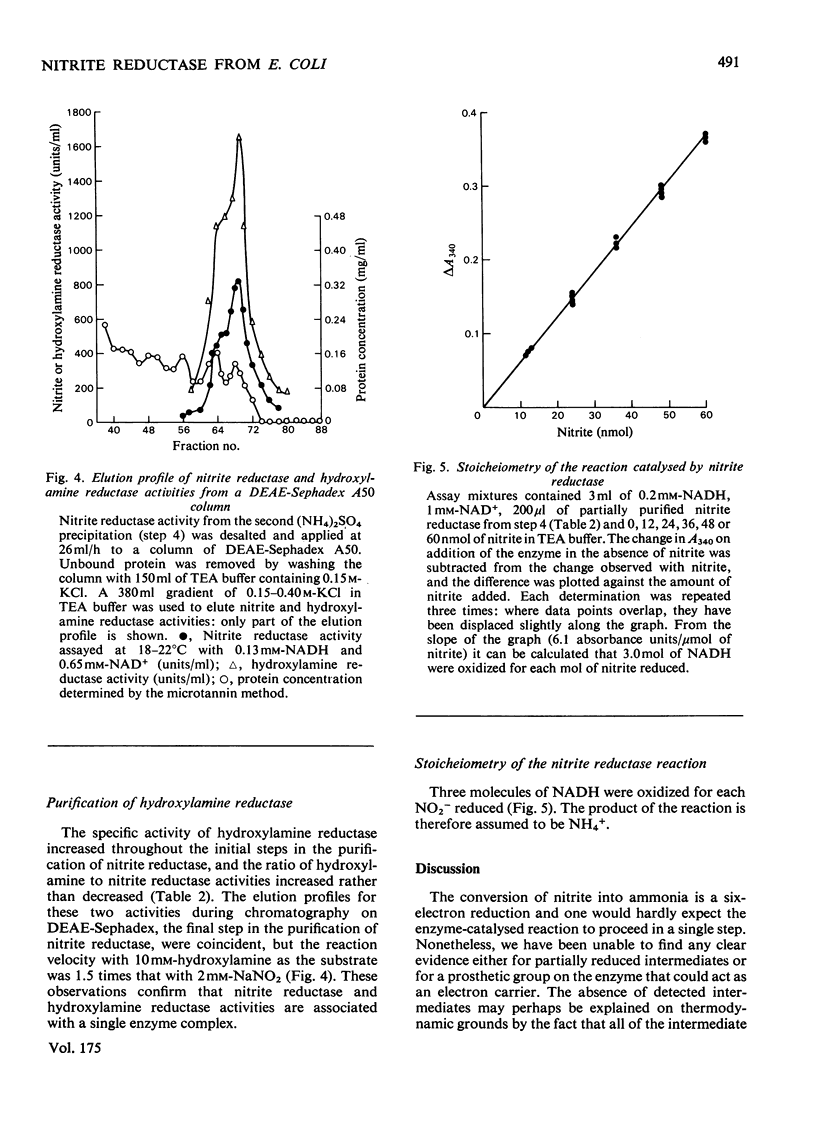


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., MCNALL E. G. Nitrate reduction. I. Growth of Escherichia coli with nitrate as sole source of nitrogen. J Bacteriol. 1956 Aug;72(2):226–229. doi: 10.1128/jb.72.2.226-229.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akroyd P. Acrylamide gel slab electrophoresis in a simple glass cell for improved resolution and comparison of serum proteins. Anal Biochem. 1967 Jun;19(3):399–410. doi: 10.1016/0003-2697(67)90229-1. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Coleman K. J., Compton B. E., Kavanagh B. M., Keevil C. W. Nitrite and ammonia assimilation by anaerobic continuous cultures of Escherichia coli. J Gen Microbiol. 1974 Nov;85(1):11–22. doi: 10.1099/00221287-85-1-11. [DOI] [PubMed] [Google Scholar]
- Cole J. A. Cytochrome c552 and nitrite reduction in Escherichia coli. Biochim Biophys Acta. 1968 Oct 1;162(3):356–368. doi: 10.1016/0005-2728(68)90122-9. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Ward F. B. Nitrite reductase-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1973 May;76(1):21–29. doi: 10.1099/00221287-76-1-21. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Activation of nitrite reductase from Escherichia coli K12 by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1978 Nov 1;175(2):495–499. doi: 10.1042/bj1750495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato R. Studies on soluble cytochromes in Enterobacteriaceae. IV. Possible involvement of cytochrome c-552 in anaerobic nitrite metabolism. J Biochem. 1966 Dec;60(6):691–700. doi: 10.1093/oxfordjournals.jbchem.a128495. [DOI] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- Hucklesby D. P., Hageman R. H. A staining method for nitrite reductase on polyacrylamide gels after electrophoresis. Anal Biochem. 1973 Dec;56(2):591–592. doi: 10.1016/0003-2697(73)90226-1. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., MASSEY V. Sedimentation studies on fumarase. Biochim Biophys Acta. 1957 Mar;23(3):544–550. doi: 10.1016/0006-3002(57)90375-x. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAREK L., MARLER E., BRADSHAW R. A., FELLOWS R. E., HILL R. L. THE SUBUNITS OF FUMARASE. J Biol Chem. 1964 Dec;239:4207–4211. [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- Kalse J. F., Veeger C. Relation between conformations and activities of lipoamide dehydrogenase. I. Relation between diaphorase and lipoamide dehydrogenase activities upon binding of FAD by the apoenzyme. Biochim Biophys Acta. 1968 Jun 4;159(2):244–256. doi: 10.1016/0005-2744(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Kemp J. D., Atkinson D. E. Nitrite reductase of Escherichia coli specific for reduced nicotinamide adenine dinucleotide. J Bacteriol. 1966 Sep;92(3):628–634. doi: 10.1128/jb.92.3.628-634.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- Lafferty M. A., Garrett R. H. Purification and properties of the Neurospora crassa assimilatory nitrite reductase. J Biol Chem. 1974 Dec 10;249(23):7555–7567. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MASSEY V., VEEGER C. Studies on the reaction mechanism of lipoyl dehydrogenase. Biochim Biophys Acta. 1961 Mar 18;48:33–47. doi: 10.1016/0006-3002(61)90512-1. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. Lack of a regulatory function for glutamine synthetase protein in the synthesis of glutamate dehydrogenase and nitrite reductase in Escherichia coli K12. J Gen Microbiol. 1977 Feb;98(2):369–377. doi: 10.1099/00221287-98-2-369. [DOI] [PubMed] [Google Scholar]
- Pateman J. A., Rever B. M., Cove D. J. Genetic and biochemical studies of nitrate reduction in Aspergillus nidulans. Biochem J. 1967 Jul;104(1):103–111. doi: 10.1042/bj1040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O. M., Sadana J. C. Purification, characterization and properties of nitrite reductase of Achromobacter fischeri. Arch Biochem Biophys. 1972 Feb;148(2):614–632. doi: 10.1016/0003-9861(72)90181-6. [DOI] [PubMed] [Google Scholar]
- Radcliffe B. C., Nicholas D. J. Some properties of a nitrite reductase from Pseudomonas denitrificans. Biochim Biophys Acta. 1968 Apr 2;153(3):545–554. doi: 10.1016/0005-2728(68)90184-9. [DOI] [PubMed] [Google Scholar]
- SPENCER D., TAKAHASHI H., NASON A. Relationship of nitrite and hydroxylamine reductases to nitrate assimilation and nitrogen fixation in Azotobacter agile. J Bacteriol. 1957 Apr;73(4):553–562. doi: 10.1128/jb.73.4.553-562.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Vega J. M., Guerrero M. G., Leadbetter E., Losada M. Reduced nicotinamide-adenine dinucleotide-nitrite reductase from Azotobacter chroococcum. Biochem J. 1973 Aug;133(4):701–708. doi: 10.1042/bj1330701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W. A., Wimpenny J. W., Cole J. A. Enzymic properties of a mutant of Escherichia coli K12 lacking nitrate reductase. Arch Mikrobiol. 1968;63(2):117–121. doi: 10.1007/BF00412166. [DOI] [PubMed] [Google Scholar]
- WALKER G. C., NICHOLAS D. J. Hydroxylamine reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:361–368. doi: 10.1016/0006-3002(61)90135-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zumft W. G. Ferredoxin:nitrite oxidoreductase from Chlorella. Purification and properties. Biochim Biophys Acta. 1972 Aug 28;276(2):363–375. doi: 10.1016/0005-2744(72)90996-5. [DOI] [PubMed] [Google Scholar]



