Abstract
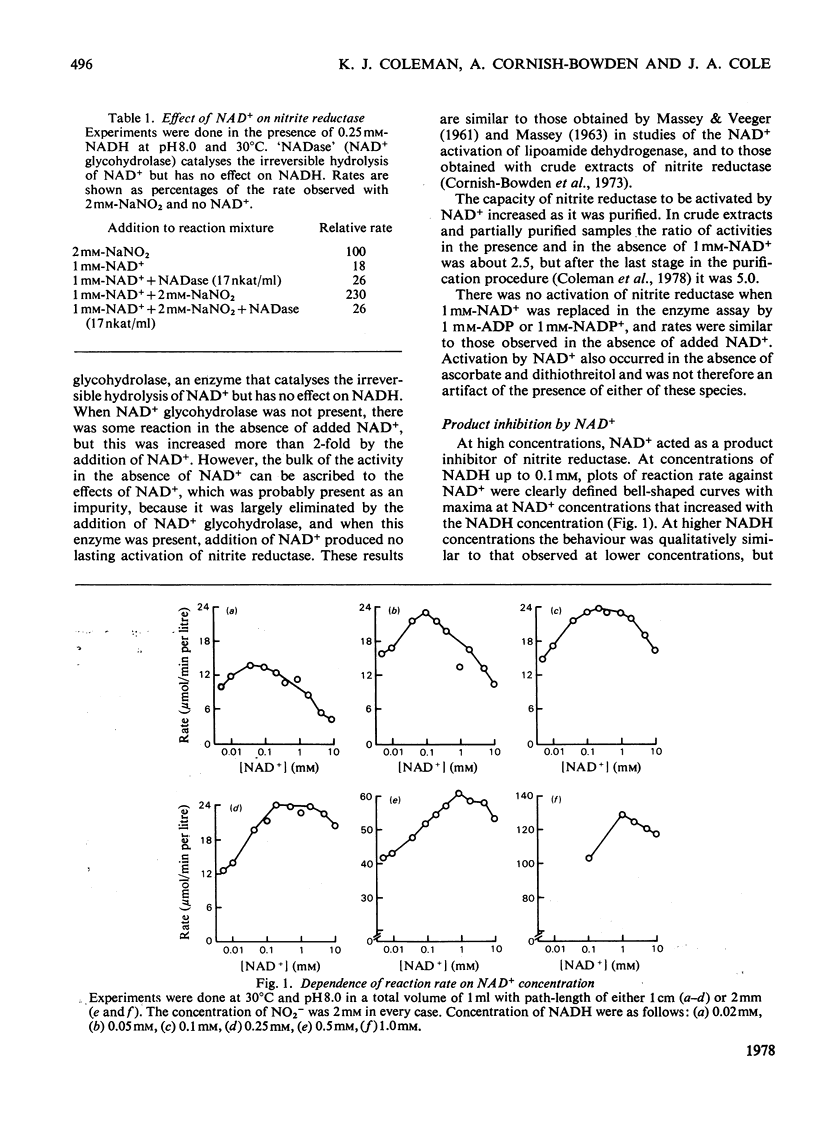
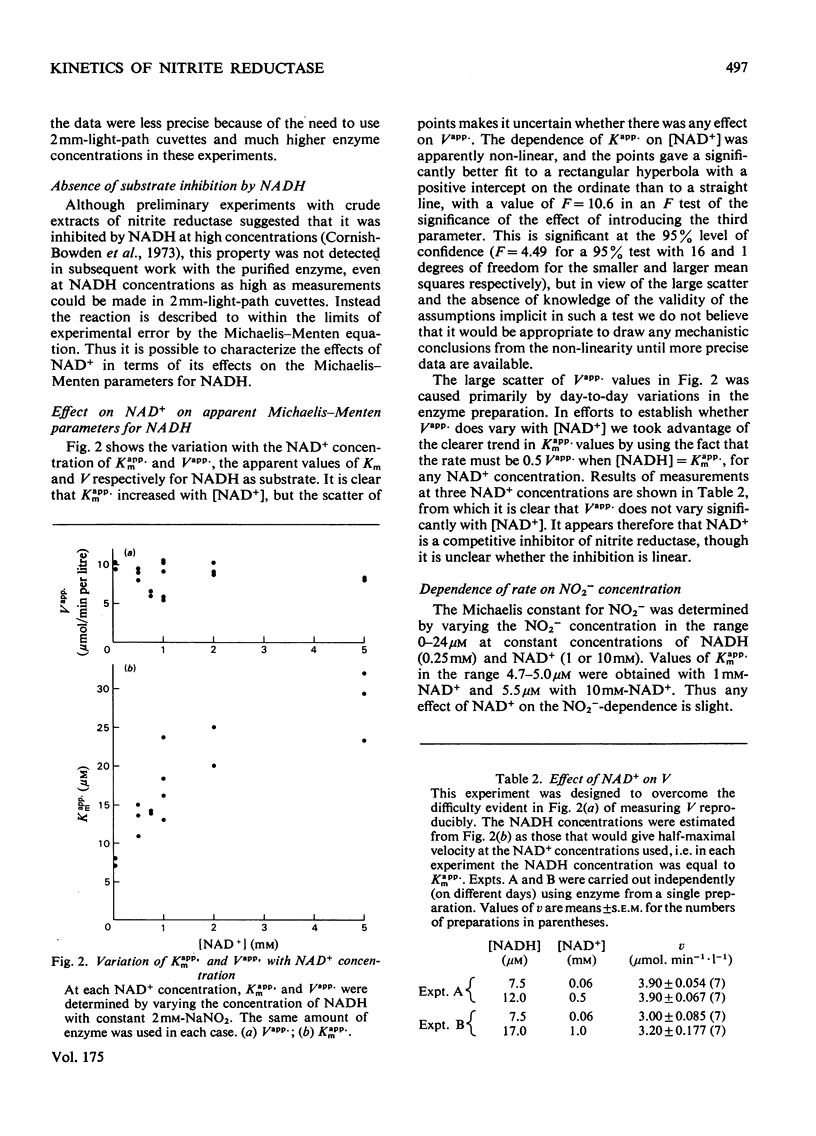
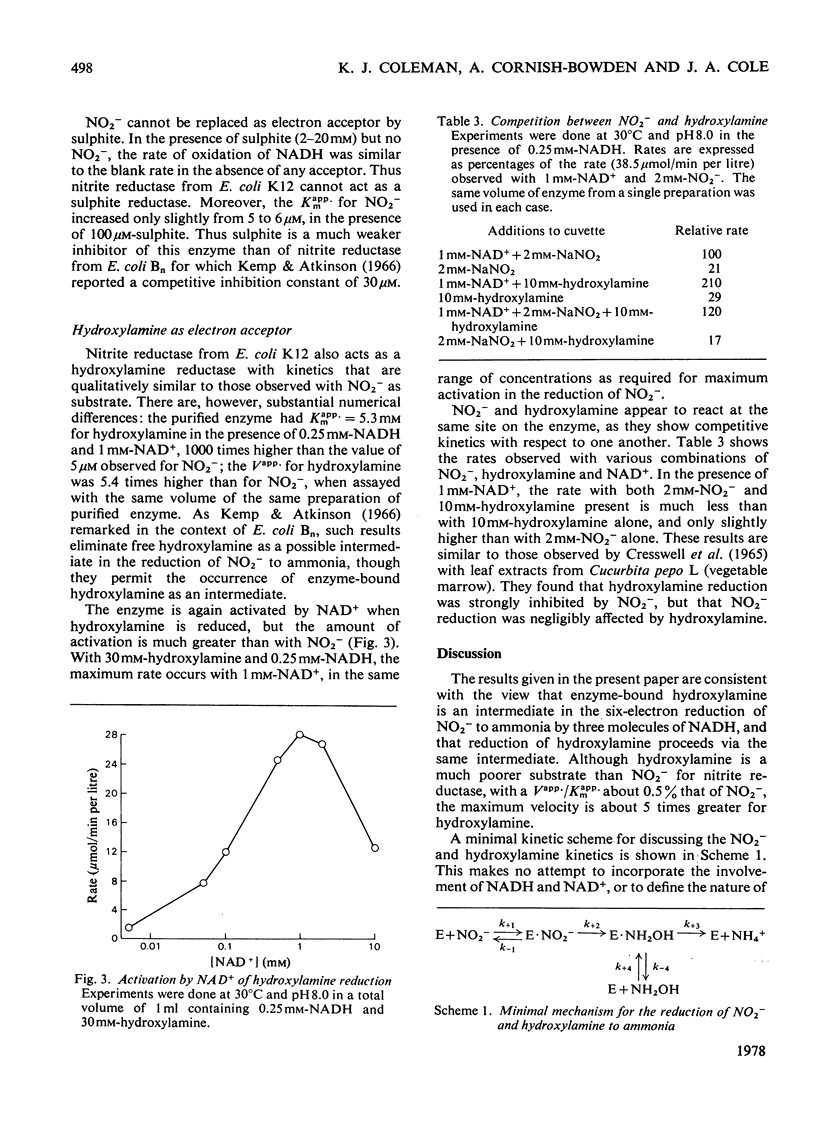
Nitrite reductase from Escherichia coli K12 requires the presence of NAD+, one of the products of the reduction of NO2-by NADH, for full activity. The effect is observed with both crude extracts and purified enzyme. NAD+ also acts as a product inhibitor at high concentrations, and plots of initial rate against NAD+ concentration are bell-shaped. The maximum occurs at about 1 mM-NAD+, but increases with increasing NADH concentration. In the presence of 1 mM-NAD+ and saturating NO2-(2mM) the Michaelis constant for NADH is about 16 micron. The Michaelis constant for NO2-is about 5 micron and is largely independent of the NAD+ concentration. Similar but more pronounced effects of NAD+ are observed with hydroxylamine as electron acceptor instead of NO2-. The maximum rate of NADH oxidation by hydroxylamine is about 5.4 times greater than the maximum rate of NADH oxidation by NO2- when assayed with the same volume of the same preparation of purified enzyme. The Michaelis constant for hydroxylamine is 5.3 mM, however, about 1000 times higher than for NO2-. These results are consistent with a mechanism in which the same enzyme-hydroxylamine complex occurs as an intermediate in both reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESSWELL C. F., HAGEMAN R. H., HEWITT E. J., HUCKLESBY D. P. THE REDUCTION OF NITRATE, NITRITE AND HYDROXYLAMINE TO AMMONIA BY ENZYMES FROM CUCURBITA PEPO L. IN THE PRESENCE OF REDUCED BENZYL VIOLOGEN AS ELECTRON DONOR. Biochem J. 1965 Jan;94:40–53. doi: 10.1042/bj0940040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978 Nov 1;175(2):483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Atkinson D. E. Nitrite reductase of Escherichia coli specific for reduced nicotinamide adenine dinucleotide. J Bacteriol. 1966 Sep;92(3):628–634. doi: 10.1128/jb.92.3.628-634.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V., VEEGER C. Studies on the reaction mechanism of lipoyl dehydrogenase. Biochim Biophys Acta. 1961 Mar 18;48:33–47. doi: 10.1016/0006-3002(61)90512-1. [DOI] [PubMed] [Google Scholar]


