Abstract
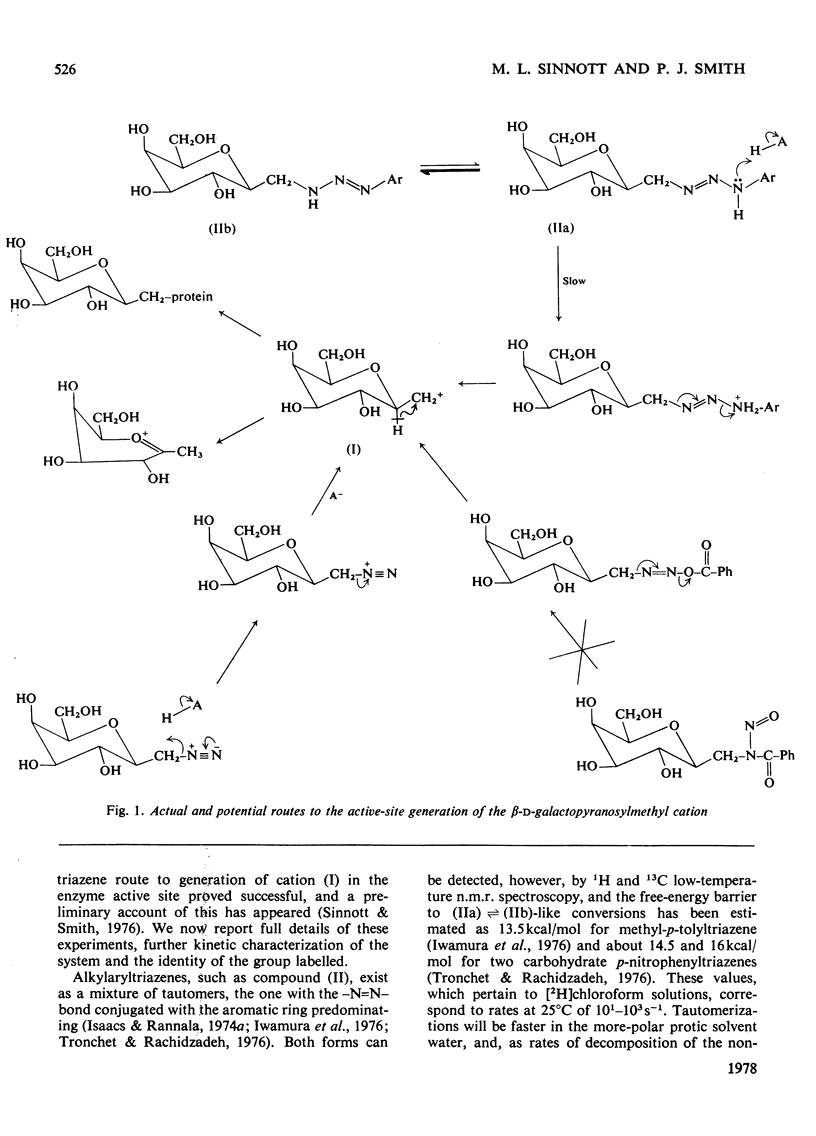
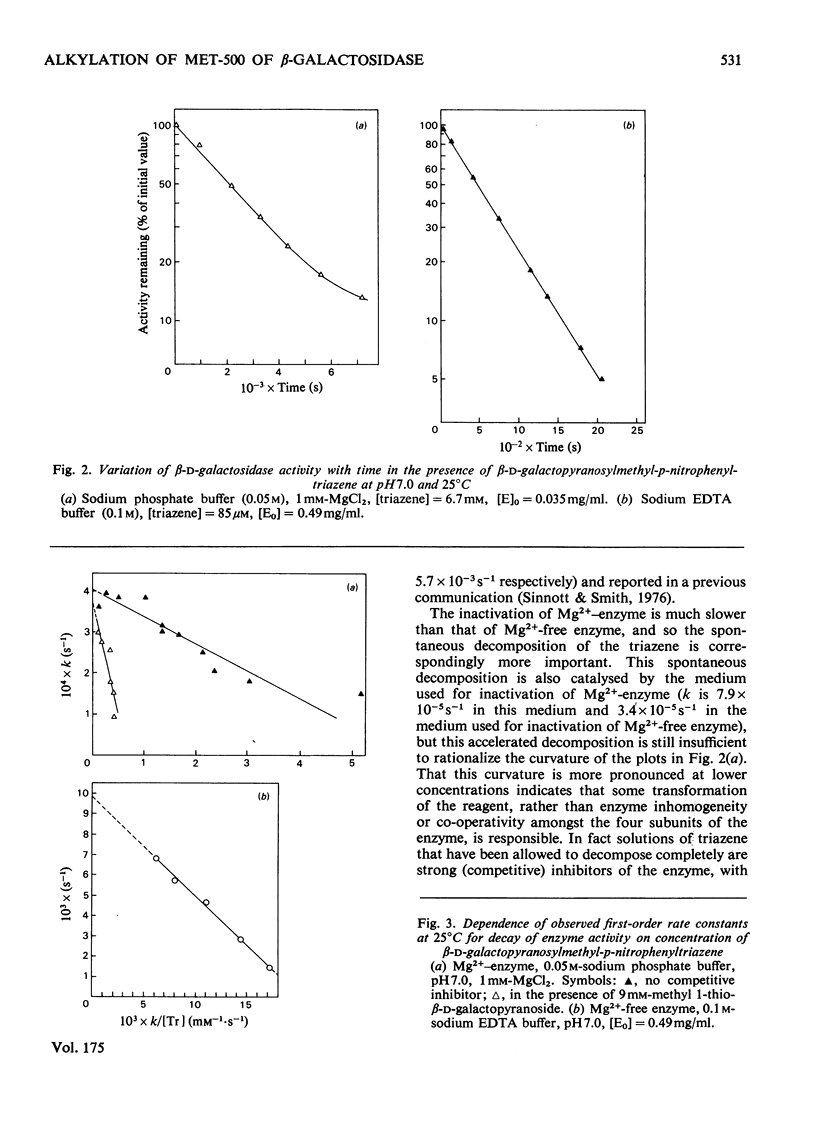
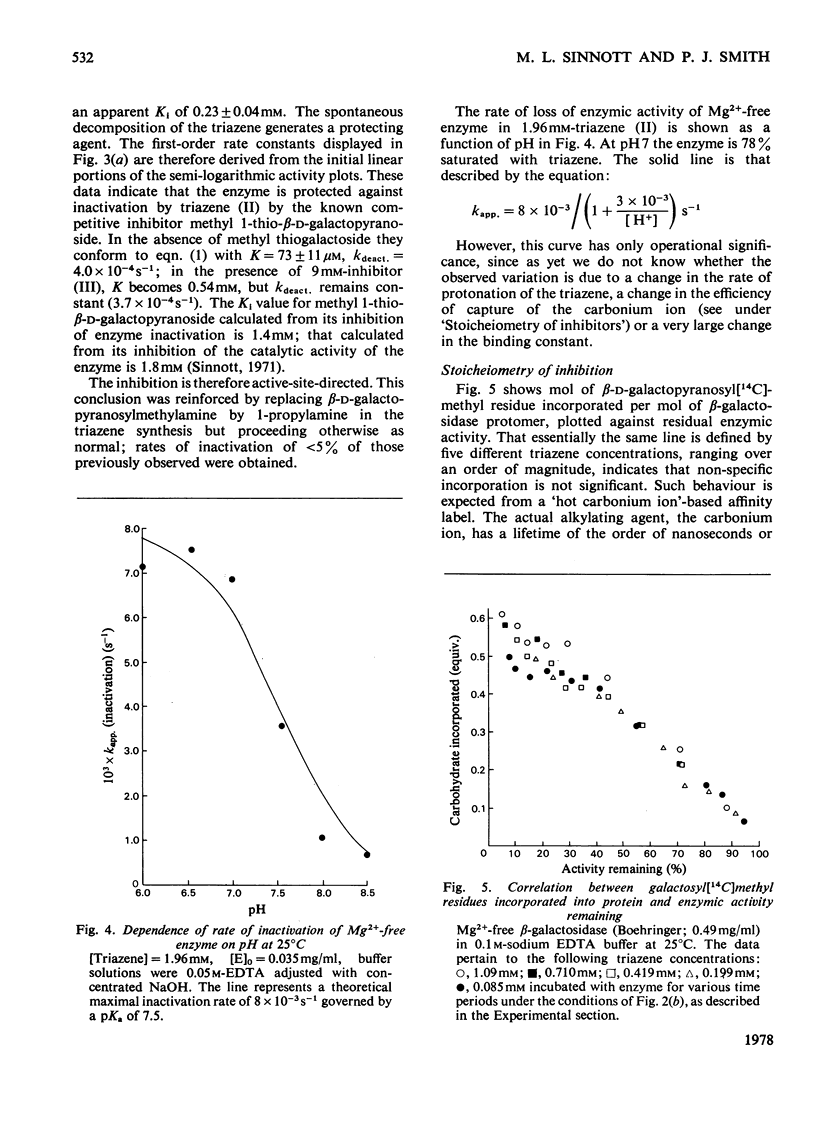
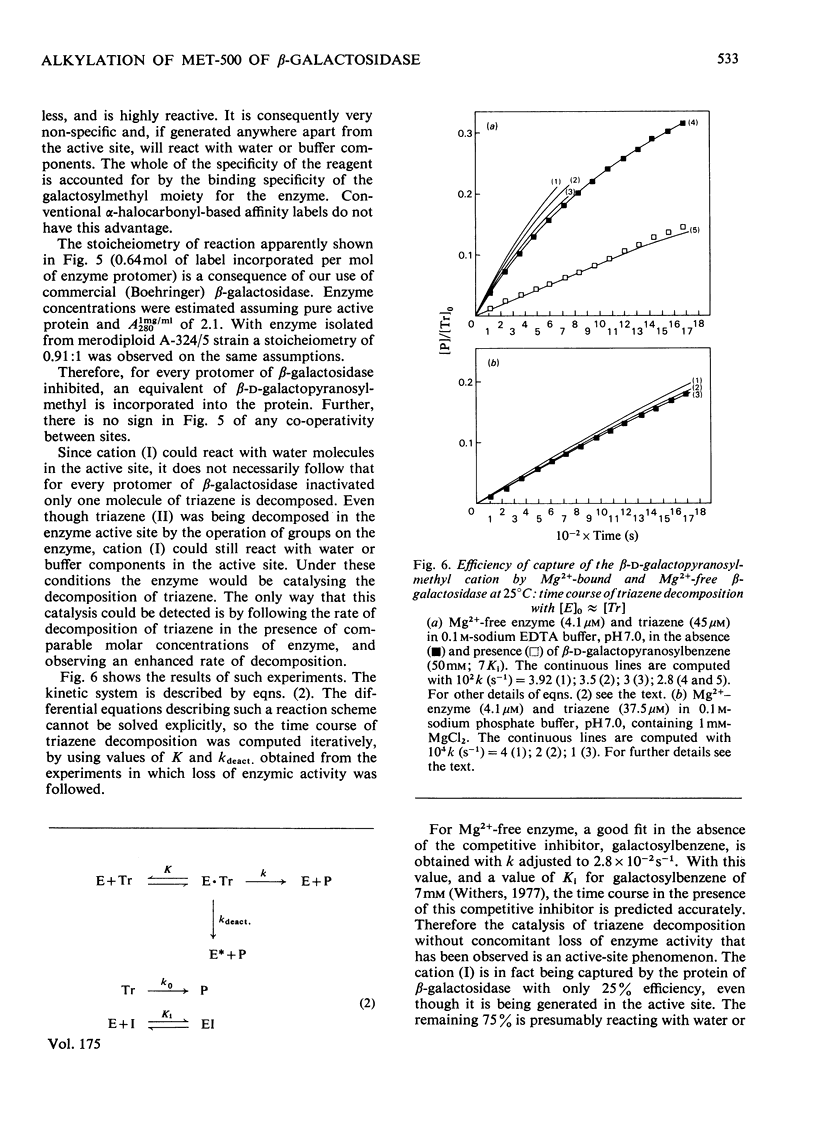
1. beta-D-Galactopyranosylmethyl-p-nitrophenyltriazene is an active-site-directed irreversible inhibitor of Mg2+-bound and Mg2+-free lacZ beta-galactosidase from Escherichia coli. 2. The Mg2+-enzyme binds the inhibitor more tightly but the complex then decomposes less rapidly than is the case with Mg2+-free enzyme. 3. Loss of enzyme activity is a linear function of the fraction of enzyme protomers to which are attached beta-D-galactopranosyl[14C]methyl residues: complete inactivation of fully active enzyme results in incorporation of 0.91 equivalent of carbohydrate label per enzyme protomer. 4. When the beta-galactopyranosylmethyl cation is generated in the active site of Mg2+-enzyme, it is captured essentially completely by the protein, but in the active site of Mg2+-free enzyme it is only captured with an efficiency of 25%. 5. Labelled enzyme was carboxymethylated and digested with trypsin; acidic hydrolysis of the isolated tryptic peptide, and field-desorption mass spectrometry of the isolated radioactive derivative, showed it to be 2,5-dioxo-3[2-(beta-D-galactopyranosylmethylthio)ethyl]-1,6-trimethylenepiperazine. 6. This is considered to have arisen from labelling of the sulphur atom of a methionine residue adjacent to a proline residue. 7. The complete amino acid sequence of the molecule [Fowler & Zabin (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 1507-1510] enables the labelled methionine residue to be identified as either Met-421 or Met-500. 8. Sequence data [Fowler, Zabin, Sinnott & Smith (1978) J. Biol. Chem. in the press] show the site of attack to be Met-500.
Full text
PDF




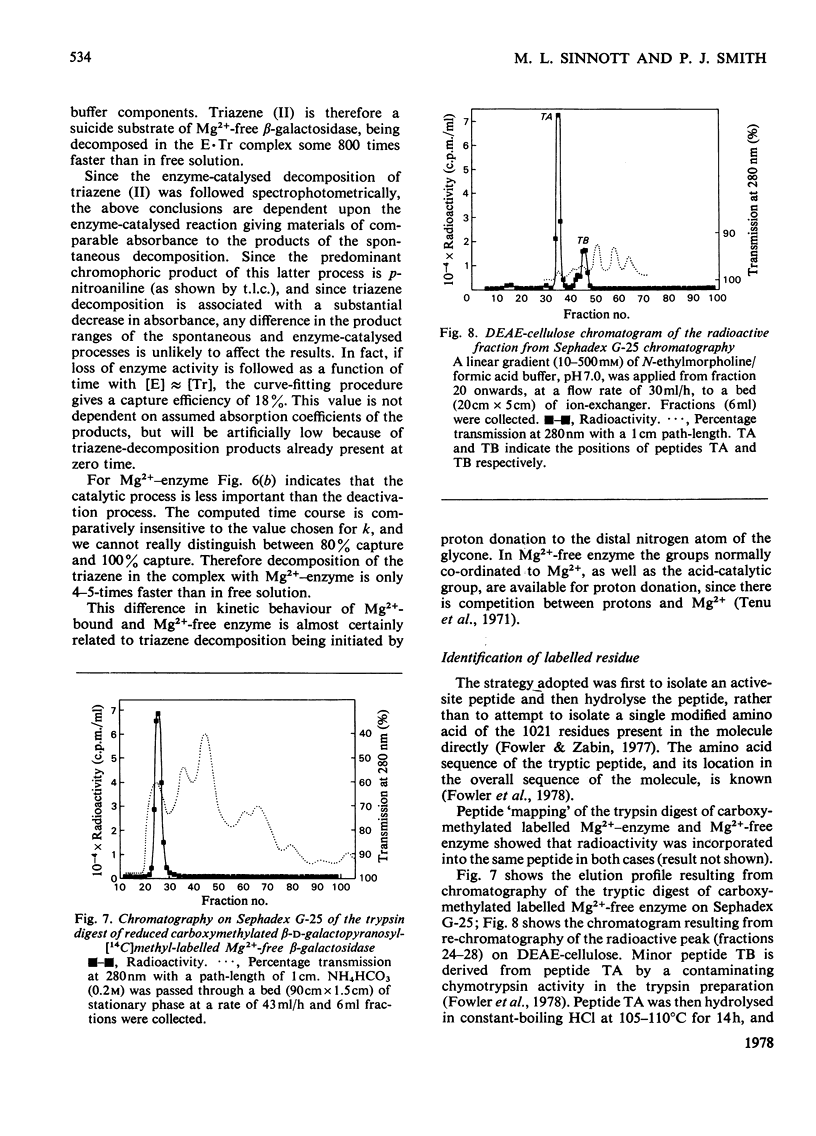
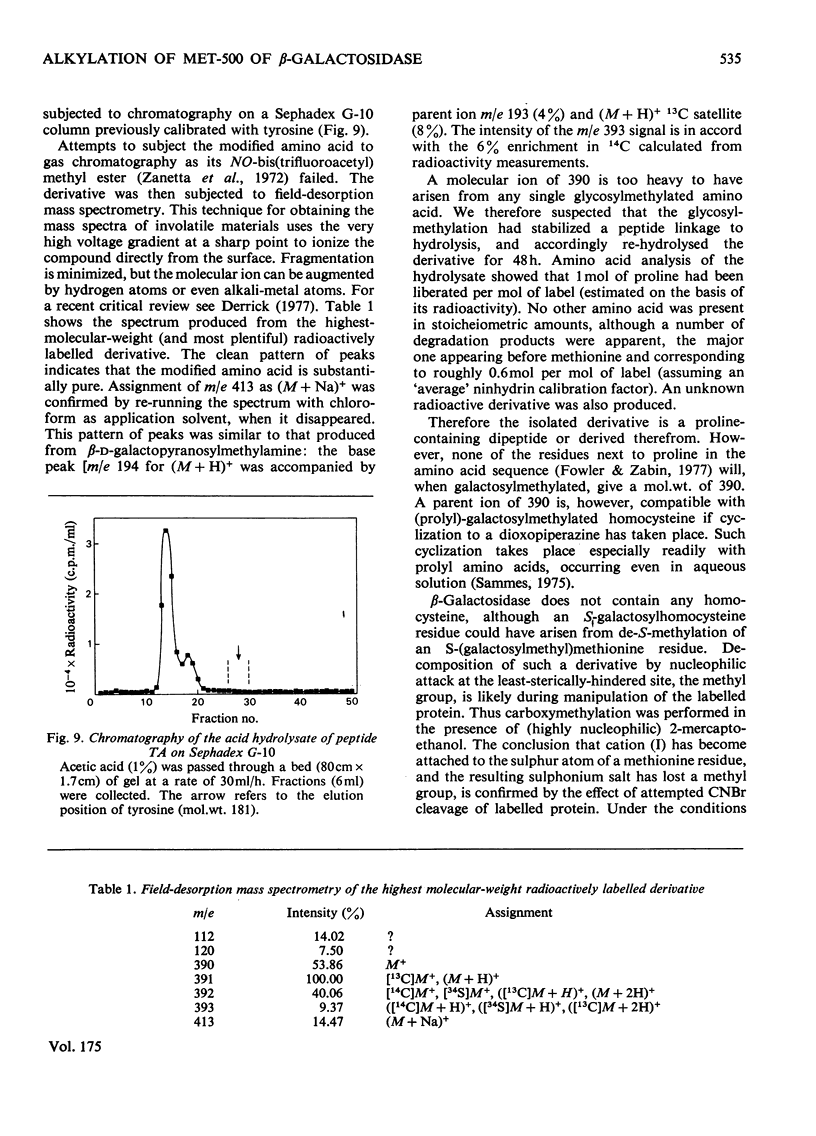
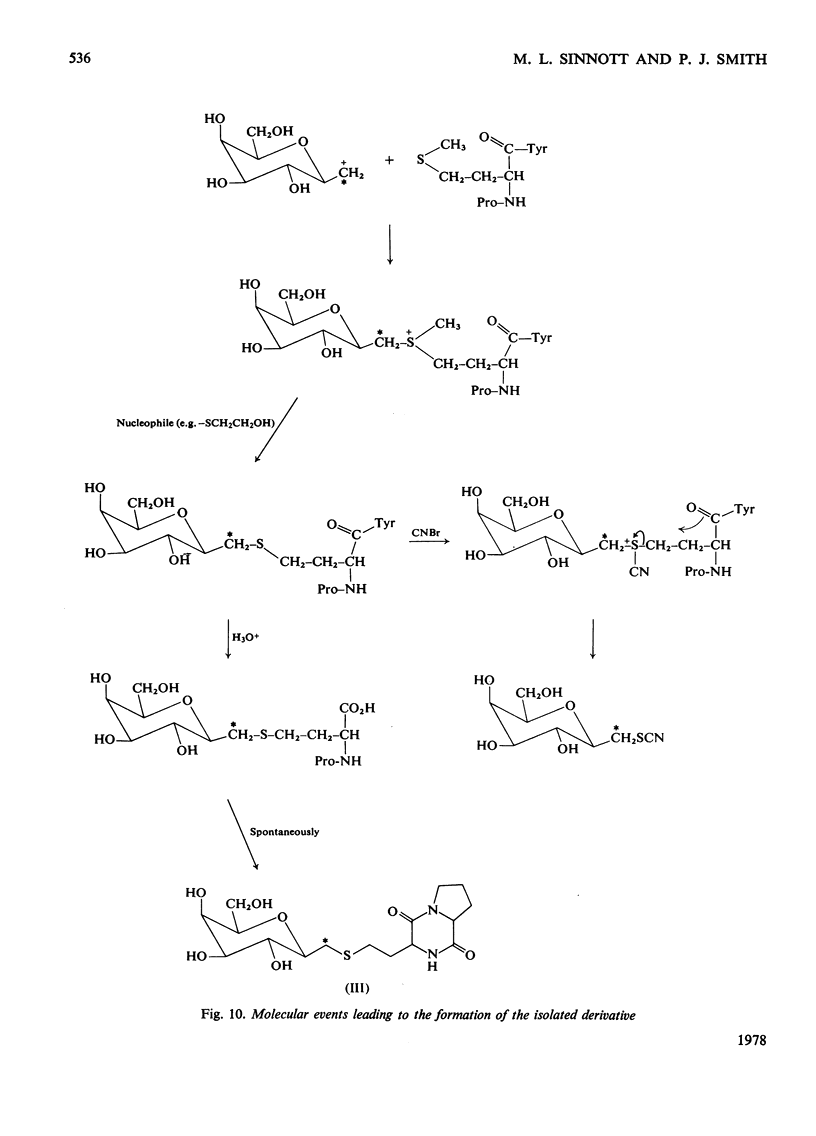


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockhaus M., Lehmann J. 2,6-anhydro-1-diazo-1-deoxy-D-glycero-L-manno-heptitol: a specific blocking agent for the active site of beta-galactosidase. FEBS Lett. 1976 Feb 15;62(2):154–156. doi: 10.1016/0014-5793(76)80041-5. [DOI] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Fowler A. V. High-level production of -galactosidase by Escherichia coli merodiploids. J Bacteriol. 1972 Nov;112(2):856–860. doi: 10.1128/jb.112.2.856-860.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Naider F., Bohak Z., Yariv J. Reversible alkylation of a methionyl residue near the active site of -galactosidase. Biochemistry. 1972 Aug 15;11(17):3202–3208. doi: 10.1021/bi00767a010. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Sammes P. G. Naturally occurring 2,5-dioxopiperazines and related compounds. Fortschr Chem Org Naturst. 1975;32:51–118. doi: 10.1007/978-3-7091-7083-0_2. [DOI] [PubMed] [Google Scholar]
- Sinnott M. L., Souchard I. J. The mechanism of action of beta-galactosidase. Effect of aglycone nature and -deuterium substitution on the hydrolysis of aryl galactosides. Biochem J. 1973 May;133(1):89–98. doi: 10.1042/bj1330089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L., Withers S. G. The beta-galactosidase-catalysed hydrolyses of beta-d-galactopyranosyl pyridium salts. Rate-limiting generation of an enzyme-bound galactopyranosyl cation in a process dependent only on aglycone acidity. Biochem J. 1974 Dec;143(3):751–762. doi: 10.1042/bj1430751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenu J. P., Viratelle O. M., Garnier J., Yon J. pH dependence of the activity of beta-galactosidase from Escherichia coli. Eur J Biochem. 1971 Jun 11;20(3):363–370. doi: 10.1111/j.1432-1033.1971.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Viratelle O. M., Yon J. Kinetic study of the activation process of -galactosidase from Escherichia coli by Mg 2+ . Eur J Biochem. 1972 Mar 15;26(1):112–118. doi: 10.1111/j.1432-1033.1972.tb01746.x. [DOI] [PubMed] [Google Scholar]
- White E. H., Roswell D. F., Politze I. R., Branchini B. R. Letter: Active site directed inhibition of enzymes utilizing deaminatively produced carbonium ions. Application to chymotrypsin. J Am Chem Soc. 1975 Apr 16;97(8):2290–2291. doi: 10.1021/ja00841a061. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]


