Abstract
Purpose
B-cell expansion with nuclear factor kappa B and T-cell anergy (BENTA) is an inborn error of immunity characterized by congenital polyclonal B-cell lymphocyte expansion. In this report, we present a case of a girl diagnosed with BENTA carrying a novel CARD11 germline mutation, aiming to clarify the clinical presentation of BENTA by conducting a literature review.
Methods
Genetic analysis, including whole-exome sequencing, was performed using genomic DNA extracted from the patient’s peripheral blood, oral mucosa, and fingernails. Additionally, a comprehensive literature review of cases with BENTA was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
A p.Leu251Pro germline variant in the CARD11 gene was identified in an 18-month-old girl with a genetic diagnosis of BENTA. She required adenoidectomy and tonsillectomy due to airway obstruction causing wheezing. Her symptoms improved with prednisolone and sirolimus. The literature review we conducted identified a total of 34 cases of BENTA. Among these cases, 15 were either asymptomatic or showed improvement without requiring any specific treatment. However, all six reported deaths were diagnosed before the age of 3 years, with two attributed to refractory hemophagocytic syndrome and four caused by opportunistic infections.
Conclusion
We present a case of BENTA with life-threatening respiratory symptoms caused by a novel CARD11 germline mutation. The patient showed a positive response to immunosuppressive therapy, including sirolimus. While BENTA is typically regarded as a benign disorder, a literature review revealed that infants with BENTA are at high risk of severe outcomes and require therapeutic intervention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-025-01872-4.
Keywords: CARD11, B-cell Expansion with Nuclear Factor Kappa B and T-cell Anergy, Sirolimus, Polyclonal B-cell Lymphocytosis, Gain-of-function Mutation
Introduction
B-cell expansion with nuclear factor kappa B (NF-κB) and T-cell anergy (BENTA) was first reported in 2012 as a rare primary immunodeficiency disorder [1]. It presents as congenital polyclonal B-cell lymphocytosis accompanied by splenomegaly, lymphadenopathy, and a reduced vaccine response. According to the recently updated 2022 classification of inborn errors of immunity by the International Union of Immunological Societies Expert Committee, BENTA is classified as a predominantly antibody deficiency [2].
The pathogenesis of BENTA involves the hyperactivation of NF-κB and mammalian target of rapamycin (mTOR) signal pathways, which is caused by heterozygous germline mutations in the CARD11 gene [1]. Upon recognition of antigens by the B-cell and T-cell receptors, CARD11 triggers the activation of the NF-κB pathway by forming the CARD11-BCL10-MALT1 signalosome [3–5]. This pathway activation leads to the polyclonal proliferation of predominantly naïve B cells. Somatic mutations in the CARD11 gene can result in lymphoid malignancies, such as activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL) [6, 7].
Patients with BENTA are at an increased risk of respiratory infections due to their immunodeficiency, leading to recurrent respiratory tract infections, sinusitis, otitis media, and viral infections, including Epstein-Barr virus [8]. Also, they may experience complications related to autoimmune diseases, such as autoimmune neutropenia and autoimmune hemolytic anemia [9]. Furthermore, patients with BENTA may exhibit poor responsiveness to immunizations, including the pneumococcal capsular polysaccharide vaccine, as well as the measles, rubella, and varicella vaccines [1]. While the polyclonal B-cell lymphocytosis in patients with BENTA generally improves with age, life-threatening events can occur during childhood, including refractory hemophagocytic lymphohistiocytosis, opportunistic infections, and complications related to immunosuppressive therapy [10].
In this report, we present a case of BENTA with a novel pathogenic germline variant in the CARD11 gene who received immunosuppressive therapy. In addition, our aim was to compare this case with previous cases by conducting a literature review in order to identify potential prognostic factors for BENTA.
Methods
Whole-Exome Sequencing
Genomic DNA was extracted from peripheral blood cells using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). The extracted DNA was then captured using SureSelect Human All Exon 50 M, V5 Kits (Agilent Technologies, Santa Clara, CA, USA). For sequencing, the captured DNA underwent massive parallel sequencing using a HiSeq 2500 next-generation sequencer (Illumina, San Diego, CA, USA), with a 100 × 2 paired-end option. The germline mutations were identified using a pipeline specifically designed for whole-exome sequencing (WES; Genomon: http://genomon.hgc.jp/exome/), as previously described [11].
Sanger Sequencing
Polymerase chain reaction amplification of the CARD11 gene was performed using PrimeSTAR GXL DNA polymerase (Takara Bio Inc., Kusatsu, Japan), the forward primer 5′-ATCTCTGCCCATCACCAACTCC-3′, and the reverse primer 5′-CCTCCTTGTAGCGTCTGACGAT-3′ for subsequent Sanger sequencing. DNA samples were extracted from various sources, including the patient’s peripheral blood, buccal mucosa, and left toenail, as well as the peripheral blood of both the patient’s father and mother. Prior to conducting the genomic testing, written informed consent was obtained from the patient’s guardians.
Structural Modeling
The three-dimensional structure of the full-length human wild-type CARD11 protein was predicted using AlphaFold2 [12]. The model, designated as AF-Q9BXL7-F1-v4, was obtained from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/entry/Q9BXL7, last updated in November 2022). The corresponding PDB file was analyzed using the PyMOL Molecular Graphics System, Version 2.5.4 (Schrödinger, Inc., LLC, New York, NY, USA). This computationally predicted structure served as the basis for homology modeling to generate the mutant CARD11 protein.
Literature Review
A systematic literature review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines using a combination of controlled vocabulary, when applicable, and keywords. The literature search was conducted on October 30, 2023, in the PubMed database (https://pubmed.ncbi.nlm.nih.gov) and included articles published up until October 2023. The search terms were “B-cell expansion with NF-κB and T-cell anergy”[TIAB] OR “BENTA”[TIAB] OR (“CARD Signaling Adaptor Proteins”[MH] AND (“lymphocytosis”[TIAB] OR “Lymphoproliferative”[TIAB] OR “B-Lymphocytes”[TIAB] OR “Primary Immunodeficiency Diseases”[MH] OR “Gain of Function”[TIAB] OR “germline”[TIAB])) AND Humans[MH] AND English[LA] AND (“0000”[PDAT]: “2023/10/30”[PDAT]).
Results
Case Report
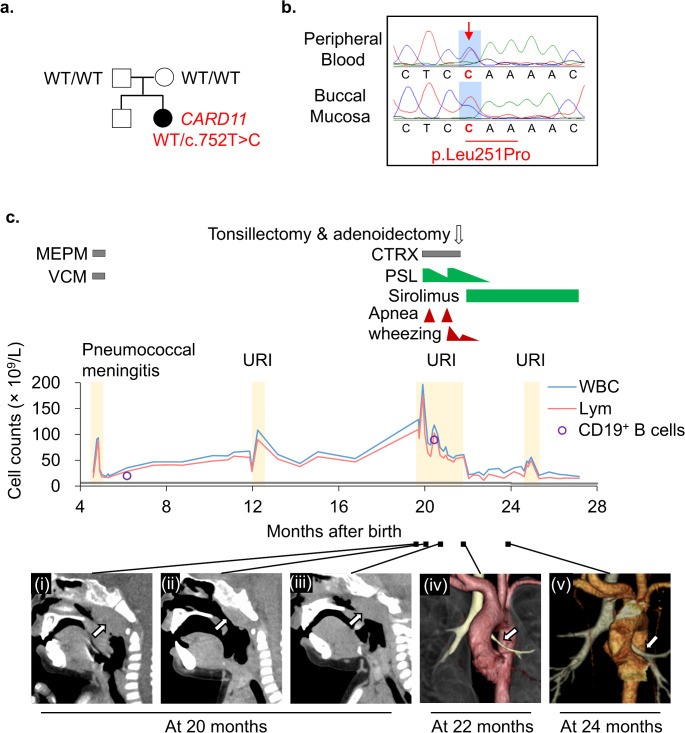
A 5-month-old infant presented to the hospital due to leukocytosis. The infant was delivered vaginally at 39 3/7 weeks gestation, weighing 3,030 g. At 4 months of age, the patient experienced bacterial meningitis caused by Streptococcus pneumoniae serotype 35B, which is not covered by the 13-valent pneumococcal conjugate vaccine. The patient received appropriate antimicrobial treatment and did not exhibit any apparent neurological complications. However, the patient was referred to the hospital due to the persistent leukocytosis of 35.5 × 109/L. The patient did not have a family history of immunodeficiency diseases or genetic disorders. During the physical examination, mild splenomegaly was observed, but no palpable lymphadenopathy was detected. Blood tests revealed an elevated white blood cell (WBC) count of 35.5 × 109/L, primarily consisting of B-lymphocytes. Additionally, mild anemia was detected with a hemoglobin level of 104 g/L, while the platelet count was 308 × 109/L (Table S1). The serum IgG level was found to be elevated at 16.43 g/L. Flow cytometric analysis revealed an increase in CD19+ B cells, particularly in the naïve B-cell subset (CD19+CD20+CD27−IgD+), without an elevation in double-negative T cells (CD3+TCR-αβ+CD4−CD8−) (Table S1). Southern blotting of the IGH gene rearrangement demonstrated a polyclonal B-cell lymphocyte proliferation pattern (Fig. S1a). Analysis of the bone marrow aspirate revealed normal trilineage blood cell differentiation with no presence of immature blasts but an increased count of normal lymphocytes (Fig. S1b). WES identified a heterozygous missense variant in the CARD11 gene (NM_032415, c.752T > C, p.Leu251Pro; Fig. 1a,b and S2a). Sanger sequencing of proband and family samples confirmed the identified variant as a de novo germline variant. Based on this finding, the patient was diagnosed with BENTA, characterized by a gain-of-function germline mutation in the CARD11 gene. Without treatment, the patient’s polyclonal B-cell lymphocytosis continued to worsen. As a result, at 20 months of age, the patient was hospitalized for an upper respiratory tract infection and wheezing, which was attributed to marked adenoid hypertrophy (Fig. 1c). The patient’s WBC count was markedly elevated at 196.9 × 109/L, and she experienced nasal congestion and apnea during sleep, necessitating the use of oxygenation. To address these symptoms, prednisolone (1.5 mg/kg/day) was administered, resulting in the shrinkage of enlarged tonsils and a decrease in the WBC count. However, as the prednisolone dosage was tapered, the patient’s respiratory symptoms worsened once again. As a result, she underwent an adenoidectomy and right tonsillectomy, which successfully resolved the wheezing. Unfortunately, 1 month later, the wheezing recurred, and the lymphocyte count increased once more. A chest computed tomography (CT) scan revealed bronchial stenosis and mediastinal lymphadenopathy caused by peribronchial tissue proliferation. The patient’s treatment was modified to include sirolimus at a dosage of 1 mg/m2/day, along with the gradual withdrawal of prednisolone. After 2 months, the patient’s wheezing completely disappeared, and subsequent CT scans revealed improvement in the bronchial stenosis and peribronchial tissue proliferation, with only minimal changes observed in the mediastinal lymph nodes and spleen. Thereafter, it is worth noting that the patient’s respiratory status remained stable with sirolimus. At 24 months of age, the patient encountered an upper respiratory tract infection and transient leukocytosis, with a maximum WBC count of 55.5 × 109/L, which improved spontaneously without the development of bronchial stenosis. Over the subsequent months, the spleen size gradually decreased, and by 10 months after the initiation of sirolimus, the splenomegaly was no longer palpable. The patient was maintained on sirolimus monotherapy.
Fig. 1.
Patient characterization. (a) The family pedigree. A heterozygous c.752T > C, p.Leu251Pro variant was identified by whole-exome sequencing. The variant was not present in the unaffected parents. (b) Sanger sequencing of the CARD11 variant in the proband. (c) Presentation of the clinical course. The patient had persistently elevated leukocytic and lymphocyte counts, which increased sharply following an upper respiratory tract infection, resulting in apneic attacks and wheezing. The gray line at the lower end of the graph indicates the age-related upper limits for absolute lymphocyte counts. Administration of prednisolone (PSL), followed by tonsillectomy and adenoidectomy, improved the apneic attacks. Subsequent treatment with sirolimus led to further reductions in white blood cell count and improvements in wheezing. (i) Computed tomography (CT) scans revealing upper airway stenosis caused by adenoid hypertrophy triggered by respiratory infection. (ii) Temporary shrinkage was observed with PSL treatment. (iii) However, the condition worsened again after PSL tapering. (iv) Three-dimensional CT imaging of the tracheobronchial tree. The left bronchus (arrow) shows stenosis due to peribronchial tissue proliferation. (v) This stenosis improved after sirolimus treatment. WT, wild-type; MEPM, meropenem; VCM, vancomycin; CTRX, ceftriaxone; PSL, prednisolone; URI, upper respiratory tract infection; WBCs, white blood cells; Lym, lymphocytes
Literature Review
During the literature search, 229 records were initially identified. Out of these, 52 reports were selected for further screening based on their titles and abstracts. Additionally, 50 reports were identified for retrieval to examine their full texts. After the screening and retrieval process, 15 eligible articles were selected, and a manual literature search identified one additional article. Thus, a total of 16 eligible articles in English were included, which provided information on 34 patients (Table S2 and Fig. 2a) [1, 9, 10, 13–25]. Detailed procedures for the literature review are described in Fig. S3. The median age at the time of diagnosis among the 34 patients was 5 years (range: 0–80 years), including our patient. Among these patients, data on CD19+ B cells were available for 30 patients, with a median CD19+ B-cell count of 6.9 × 109/L (range: 0.6–48.2 × 109/L). The distribution of age and B-cell count among the patients is depicted in Fig. 2b, while the locations of the CARD11 gene mutation are illustrated in Fig. S2b. Out of the 35 patients, 15 presented with mild symptoms or were asymptomatic and did not require immunoglobulin replacement therapy, splenectomy, or immunosuppressive therapy. Among the 21 patients who were 3 years old or older at the time of diagnosis, all achieved long-term survival. However, among the 14 patients who were younger than three years old at diagnosis, six died within 3–9 years of diagnosis. Two of these deaths were due to refractory hemophagocytic lymphohistiocytosis, while the remaining four were caused by opportunistic infections.
Fig. 2.
Clinical course of patients with B-cell expansion with nuclear factor kappa B and T-cell anergy (BENTA) identified from a literature review. (a) The swimmer plot illustrates the treatment courses of 34 patients with BENTA. (b) The distribution of age and B-cell count. The gray boxes represent the normal age-related ranges for absolute CD19+ B-cell counts. Out of the 16 patients who were younger than 3 years at the time of diagnosis, six died within 3–9 years following their diagnosis due to refractory hemophagocytic lymphohistiocytosis or opportunistic infections. UPN, unique patient number; SCIG, subcutaneous immunoglobulin; IVIG, intravenous immunoglobulin
Four patients underwent sirolimus treatment. In two cases, the patients were initially suspected of autoimmune lymphoproliferative syndrome because of B-cell proliferation, splenomegaly, anemia, thrombocytopenia, and recurrent respiratory infections [9, 10]. Sirolimus effectively reduced the size of palpable lymph nodes and spleen in these two patients. In another two cases, the patients had concomitant severe multisystem autoimmune diseases [9, 10]. Immunosuppressive combination therapy, including steroids, rituximab, and sirolimus, was employed to treat cytopenia. Despite initial improvement, the course of treatment eventually failed to control the progression of the disease in these two patients.
Two patients underwent allogeneic hematopoietic stem cell transplantation (HSCT). The first patient was a 3-month-old boy who received HSCT from a matched, unrelated donor to address severe immune dysregulation, including cytopenia, granulomatous lymphocytic interstitial lung disease, and nephrotic syndrome [10]. However, he experienced primary graft failure. Consequently, he received a second transplant from the same donor, but unfortunately, he again experienced graft failure and ultimately died due to an adenovirus infection. The second case involved an adult male who received a matched HSCT from his sister to treat chronic lymphocytic leukemia, which developed around the age of 44 [1]. Prior to the diagnosis, he had experienced persistent benign B-cell lymphocytosis since infancy. In adulthood, he presented with a persistent upper respiratory illness and pleural effusion. Following the HSCT, he has remained disease-free for five years.
Discussion
We present a case of a 5-month-old patient with BENTA, which is caused by a germline missense variant (p.Leu251Pro) in the CARD11 gene. This condition was effectively managed with sirolimus. Germline gain-of-function CARD11 mutations are known to cause BENTA, while somatic gain-of-function CARD11 mutations are frequently observed in patients with ABC DLBCL, which is an aggressive and intractable subtype of DLBCL. The p.Leu251Pro variant, located in the Coiled-Coil domain of the CARD11 protein (Fig. S2a), was first identified and confirmed as a gain-of-function mutation in patients with ABC DLBCL [6]. The gain-of-function CARD11 mutations in the Coiled-Coil domain activate NF-κB signaling by inducing spontaneous aggregation of the CARD11-BCL10-MALT1 complex, independent of cell-surface antigen receptor-stimulated phosphorylation [6, 26]. The p.Leu251Pro variant has been demonstrated to activate the NF-κB and mTOR pathways in experimental studies using transgenic cell lines [27]. The Catalogue of Somatic Mutations in Cancer database (https://cancer.sanger.ac.uk/cosmic, accessed on October 30, 2023) lists the p.Leu251Pro variant as a somatic mutation found in 14 cases of malignant lymphomas (12 DLBCL and two follicular lymphomas). Although this variant has been reported somatically, our case presents the first known germline occurrence of the p.Leu251Pro variant. We made a diagnosis of BENTA in the proband based on the criteria established by the American College of Medical Genetics and Genomics guideline (PS2, PS3, PM1, PM2, PP2, and PP4) [28].
The gain-of-function mutations in the CARD11 gene result in the constitutive activation of the NF-κB signaling pathway, leading to abnormal proliferation and enhanced survival of immature B cells, with variable consistency among patients [1, 6, 10, 13, 17, 18, 22, 23, 25, 29]. These mutations also independently activate mTOR, as demonstrated in studies involving DLBCL cell lines with the p.Leu251Pro variant [27] and in vitro analyses of the cells of patients with BENTA [21]. Remarkably, mTOR inhibition has been demonstrated to suppress germinal center responses in peripheral B cells, emphasizing its potential therapeutic utility in refractory B-cell lymphomas [30]. Hence, sirolimus, an mTOR inhibitor, is expected to be effective in alleviating symptoms in patients with BENTA. In previous reports, sirolimus demonstrated effectiveness in two patients by reducing adenopathy and splenomegaly, while it was found to be ineffective in two other patients [9, 10]. In the present case, treatment with sirolimus resulted in a reduction in the number of B-lymphocytes in the peripheral blood and alleviated wheezing caused by airway narrowing due to lymphoid tissue proliferation, although prednisolone, which exerts inhibitory effects on NF-κB signaling, failed to prevent disease progression.
The prognostic factors for patients with BENTA are currently unknown. However, a literature review of 34 cases revealed that all six fatal cases were diagnosed at an age of less than 3 years. This suggests that younger patients with BENTA should be managed carefully, as they may have a worse prognosis. BENTA cases can vary in severity and may not always be mild or asymptomatic. In the present case, there was a marked increase in lymphocyte counts (WBC count: 196.9 × 109/L) compared to previous reports. In the future, if clinical factors such as age and lymphocyte count are found to be prognostic, they could provide valuable insights for making important clinical decisions, including the choice of drug treatment and/or the consideration of allogeneic HSCT.
In conclusion, we diagnosed a patient with BENTA based on a novel heterozygous missense pathogenic germline variant in the CARD11 gene (p.Leu251Pro). The patient responded well to treatment with prednisone and sirolimus. It is expected that future studies, involving a larger number of cases, will help identify prognostic factors that can assist in making important clinical decisions, including the indication for pharmaceutical intervention and/or allogeneic HSCT.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to all clinicians, patients, and families, as well as Ms. Yoshie Miura, Ms. Fumiyo Ando, Ms. Hiroko Ono, and Ms. Chie Amahori for their invaluable assistance.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TN, HM, MW, and DY. Clinical information was gathered by TN, TF, YS, and YM. Data interpretation was performed by DS, RM, YT, AY, KN, SK, AN, and NN. The research was directed by YT. The first draft of the manuscript was written by TN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from Japanese Ministry of Health, Labour and Welfare (Grant number [20FC1053]) and Nagoya Pediatric Cancer Fund.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Nagoya University Graduate School of Medicine (Date 25/01/2023; Approval number TT23001).
Consent to Participate
Written informed consent was obtained from the parents.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hideki Muramatsu, Email: hideki-muramatsu@med.nagoya-u.ac.jp.
Yoshiyuki Takahashi, Email: ytakaha@med.nagoya-u.ac.jp.
References
- 1.Snow AL, Xiao W, Stinson JR, Lu W, Chaigne-Delalande B, Zheng L, et al. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med. 2012;209(12):2247–61. 10.1084/jem.20120831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2022;42(7):1473–507. 10.1007/s10875-022-01289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meininger I, Krappmann D. Lymphocyte signaling and activation by the CARMA1-BCL10-MALT1 signalosome. Biol Chem. 2016;397(12):1315–33. 10.1515/hsz-2016-0216. [DOI] [PubMed] [Google Scholar]
- 4.Lu HY, Bauman BM, Arjunaraja S, Dorjbal B, Milner JD, Snow AL, et al. The CBM-opathies-A rapidly expanding spectrum of human inborn errors of immunity caused by mutations in the CARD11-BCL10-MALT1 complex. Front Immunol. 2018;9:2078. 10.3389/fimmu.2018.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedsaul JR, Carter NM, Deibel KE, Hutcherson SM, Jones TA, Wang Z, et al. Mechanisms of regulated and dysregulated CARD11 signaling in adaptive immunity and disease. Front Immunol. 2018;9:2105. 10.3389/fimmu.2018.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–9. 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 7.Reimann M, Schrezenmeier J, Richter-Pechanska P, Dolnik A, Hick TP, Schleich K, et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood. 2021;137(20):2785–99. 10.1182/blood.2020005244. [DOI] [PubMed] [Google Scholar]
- 8.Arjunaraja S, Angelus P, Su HC, Snow AL. Impaired control of Epstein-Barr Virus infection in B-Cell expansion with NF-kappaB and T-Cell Anergy Disease. Front Immunol. 2018;9:198. 10.3389/fimmu.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta M, Aluri J, Desai M, Lokeshwar M, Taur P, Lenardo M, et al. Clinical, immunological, and Molecular findings in four cases of B cell expansion with NF-kappaB and T cell Anergy Disease for the First Time from India. Front Immunol. 2018;9:1049. 10.3389/fimmu.2018.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urdinez L, Erra L, Palma AM, Mercogliano MF, Fernandez JB, Prieto E, et al. Expanding spectrum, intrafamilial diversity, and therapeutic challenges from 15 patients with heterozygous CARD11-associated diseases: a single center experience. Front Immunol. 2022;13:1020927. 10.3389/fimmu.2022.1020927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muramatsu H, Okuno Y, Yoshida K, Shiraishi Y, Doisaki S, Narita A, et al. Clinical utility of next-generation sequencing for inherited bone marrow failure syndromes. Genet Med. 2017;19(7):796–802. 10.1038/gim.2016.197. [DOI] [PubMed] [Google Scholar]
- 12.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brohl AS, Stinson JR, Su HC, Badgett T, Jennings CD, Sukumar G, et al. Germline CARD11 mutation in a patient with severe congenital B cell lymphocytosis. J Clin Immunol. 2015;35(1):32–46. 10.1007/s10875-014-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder D, Stinson JR, Nugent DJ, Heurtier L, Suarez F, Sukumar G, et al. Mild B-cell lymphocytosis in patients with a CARD11 C49Y mutation. J Allergy Clin Immunol. 2015;136(3):819–21 e1. 10.1016/j.jaci.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Outinen T, Syrjanen J, Rounioja S, Saarela J, Kaustio M, Helminen M. Constant B cell lymphocytosis since early age in a patient with CARD11 mutation: a 20-year follow-up. Clin Immunol. 2016;165:19–20. 10.1016/j.clim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Buchbinder D, Sassoon A. A case of bad Carma! Blood. 2017;129(12):1737. 10.1182/blood-2016-12-756007. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins M, Arjunaraja S, Stinson JR, Dorjbal B, Sundaresan J, Niemela J, et al. A unique heterozygous CARD11 mutation combines pathogenic features of both gain- and loss-of-function patients in a four-Generation Family. Front Immunol. 2018;9:2944. 10.3389/fimmu.2018.02944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields AM, Bauman BM, Hargreaves CE, Pollard AJ, Snow AL, Patel SY. A Novel, heterozygous three base-pair deletion in CARD11 results in B cell expansion with NF-kappaB and T cell Anergy Disease. J Clin Immunol. 2020;40(2):406–11. 10.1007/s10875-019-00729-x. [DOI] [PubMed] [Google Scholar]
- 19.Neishabury M, Azarkeivan A, Mehri M, Najmabadi H, Cheraghi T. The First Case of BENTA Disease (B Cell expansion with NF-kappaB and T cell anergy) from Iran. J Clin Immunol. 2021;41(4):811–3. 10.1007/s10875-021-00965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludgate JL, Weeks RJ, Morison IM. Aberrant immunoglobulin Kappa Locus Rearrangement in a patient with CARD11-Related B cell lymphocytosis. J Clin Immunol. 2021;41(8):1943–5. 10.1007/s10875-021-01114-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P, Meng Q, Huang Y, Zhang L, Luo S, Zhang X, et al. Identification and characterization of a germline mutation in CARD11 from a Chinese case of B cell expansion with NF-kappaB and T cell anergy. Front Immunol. 2021;12:676386. 10.3389/fimmu.2021.676386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takase Y, Tanioka S, Ishimura M, Yoshiura KI, Mori Y, Sakaida E, et al. A familial case of B-cell expansion with NF-kappaB and T-cell anergy caused by a G123D heterozygous missense mutation in the CARD11 gene. Pediatr Blood Cancer. 2022;69(12):e29941. 10.1002/pbc.29941. [DOI] [PubMed] [Google Scholar]
- 23.Zhao P, Hu Y, Sun D, Meng Q, Zhang L, Zhang X, et al. A novel CARD11 germline mutation in a Chinese patient of B cell expansion with NF-kappaB and T cell anergy (BENTA) and literature review. Front Immunol. 2022;13:943027. 10.3389/fimmu.2022.943027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao H, Mou W, Zhang R, Guo X, Chen H, Zhang L, et al. Manifestations of B-cell expansion with NF-kappaB and T-cell anergy disease overlapping with hemophagocytic lymphohistiocytosis. Scand J Immunol. 2023;97(4):e13256. 10.1111/sji.13256. [DOI] [PubMed] [Google Scholar]
- 25.Bauman BM, Dorjbal B, Pittaluga S, Zhang Y, Niemela JE, Stoddard JL, et al. Subcutaneous panniculitis-like T-cell lymphoma in two unrelated individuals with BENTA disease. Clin Immunol. 2023;255:109732. 10.1016/j.clim.2023.109732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan W, Schaffer TB, Pomerantz JL. A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival. Mol Cell Biol. 2013;33(2):429–43. 10.1128/MCB.00850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray-Dutra MN, Chawla R, Thomas KR, Seymour BJ, Arkatkar T, Sommer KM, et al. Activated CARD11 accelerates germinal center kinetics, promoting mTORC1 and terminal differentiation. J Exp Med. 2018;215(9):2445–61. 10.1084/jem.20180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–74. 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arjunaraja S, Nose BD, Sukumar G, Lott NM, Dalgard CL, Snow AL. Intrinsic plasma cell differentiation defects in B cell expansion with NF-kappaB and T cell anergy patient B cells. Front Immunol. 2017;8:913. 10.3389/fimmu.2017.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton KS, Phong B, Corey C, Cheng J, Gorentla B, Zhong X, et al. T cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci Signal. 2014;7(329):ra55. 10.1126/scisignal.2005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.