Abstract
1. The 120-fold purification of ethanolamine ammonia-lyase from Escherichia coli extracts, to apparent homogeneity, is described. Ethanolamine, dithiothreitol, glycerol and KCl protected the apoenzyme from inactivation. 2. At the optimum pH7.5, Km values for ethanolamine and coenzyme B12 were 44μm and 0.42μm respectively. The Km for ethanolamine was markedly affected by pH, transitions occurring at pH7.0 and 8.35. 3. The enzyme was specific for ethanolamine as substrate, none of the 18 analogues tested being active. l-2-Aminopropan-l-ol (Ki 0.86μm), dl-1-aminopropan-2-ol (Ki 2.2μm) and dl-1,3-diaminopropan-2-ol (Ki 88.0μm) inhibited competitively. 4. Enzyme activity was inhibited, irreversibly and non-competitively, by the coenzyme analogues methylcobalamin (Ki 1.4nm), hydroxocobalamin (Ki 2.1nm) and cyanocobalamin (Ki 4.8nm). 5. Iodoacetamide inhibited in the absence of ethanolamine, but only slightly in its presence. p-Hydroxymercuribenzoate inhibited markedly even in the presence of ethanolamine. Dithiothreitol and 2-mercaptoethanol (less effectively) restored activity to the enzyme dialysed against buffer containing ethanolamine. 6. Although K+ ions stabilized the enzyme during dialysis or storage, they were not necessary for activity. 7. Gel filtration showed the enzyme to be of high molecular weight, ultracentrifugal studies giving s20,w of 16.4 and an estimated mol.wt. 560400. The isoelectric point for the apoenzyme was approx. pH5.0. inhibited enzyme activity at concentrations above 1m (95% inhibition at 3m) and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis indicated protein subunits of mol.wt. 61400. 8. Immunological studies showed that the E.coli enzyme was closely related to those of other enterobacteria, but only distantly to that of Clostridium sp. A double precipitin band suggested that the apoenzyme may be made up of two protein components.
Full text
PDF


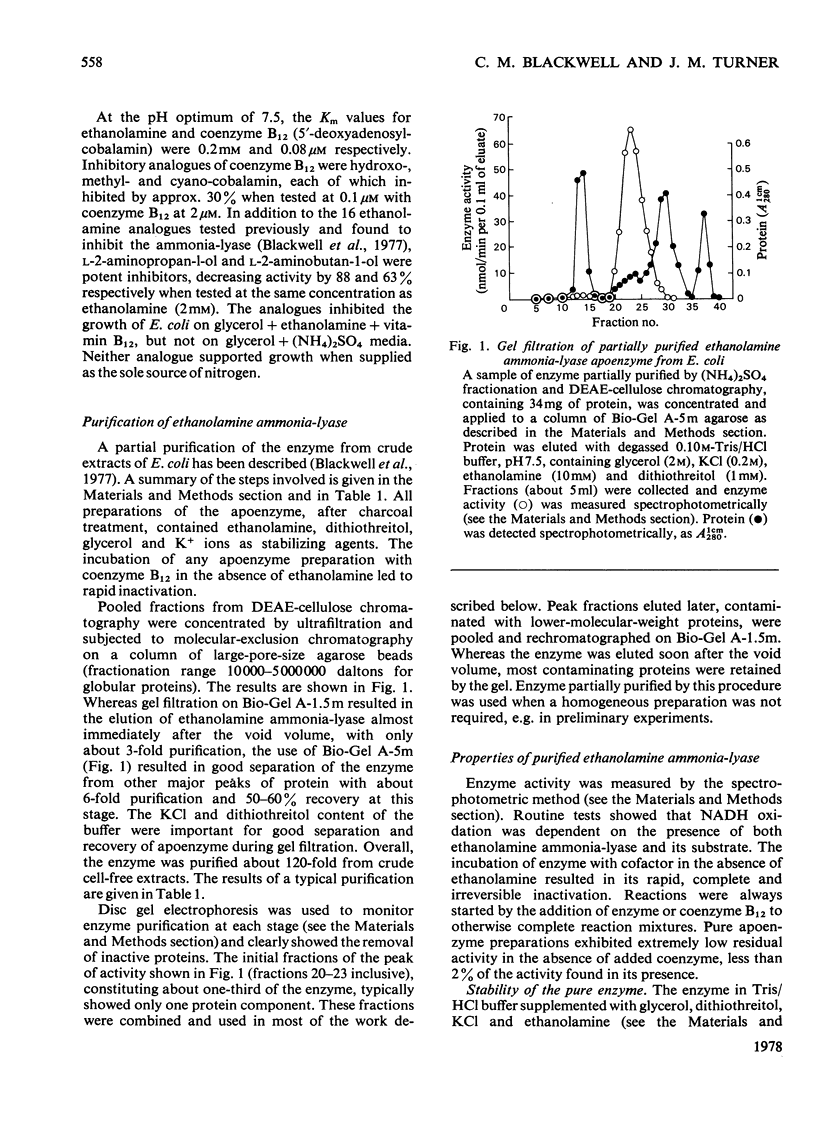

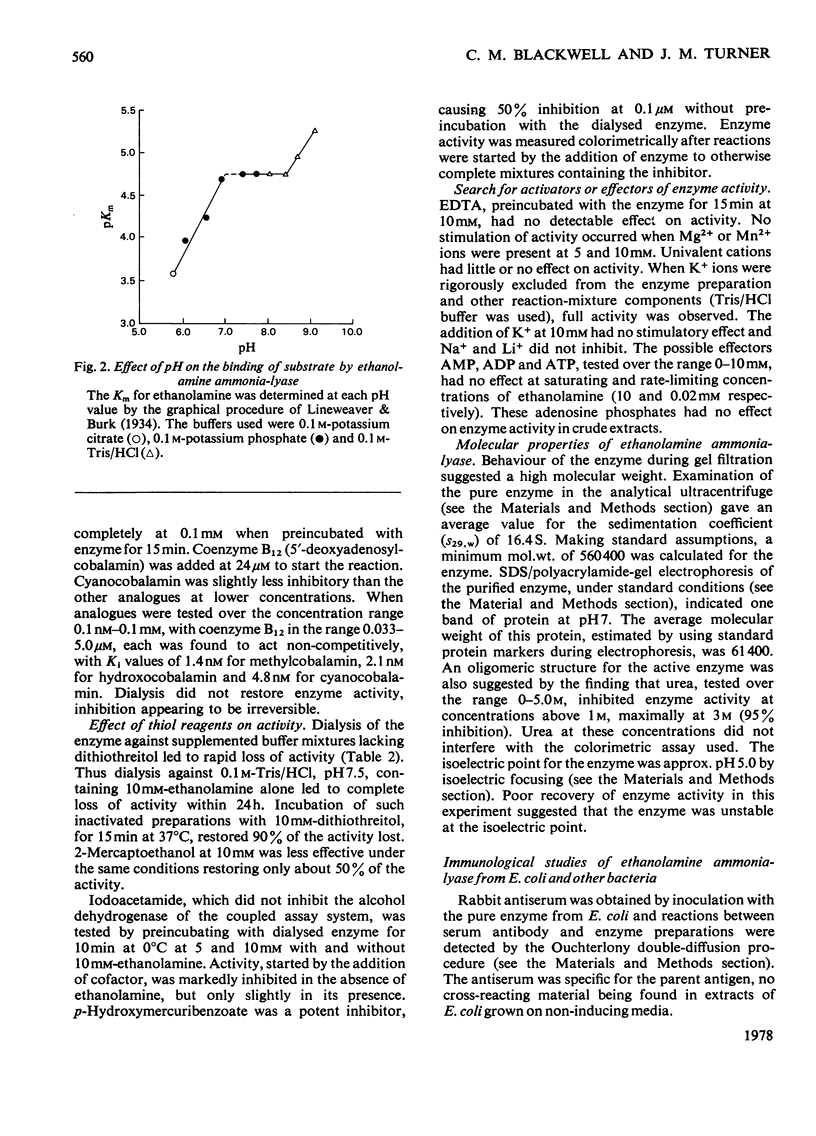



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Blackwell C. M., Scarlett F. A., Turner J. M. Ethanolamine catabolism by bacteria, including Escherichia coli. Biochem Soc Trans. 1976;4(3):495–497. doi: 10.1042/bst0040495. [DOI] [PubMed] [Google Scholar]
- Blackwell C. M., Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Control of formation and stability of partially purified ethanolamine ammonia-lyase in Escherichia coli. J Gen Microbiol. 1977 Jan;98(1):133–139. doi: 10.1099/00221287-98-1-133. [DOI] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Chang G. W., Chang J. T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- Clough H. B., Shukla S. D., Turner J. M. Biosynthetic utilization of ethanolamine by Erwinia carotovora. Biochem Soc Trans. 1975;3(5):769–772. doi: 10.1042/bst0030769. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- HOGENKAMP H. P., LADD J. N., BARKER H. A. The identification of a nucleoside derived from coenzyme B12. J Biol Chem. 1962 Jun;237:1950–1952. [PubMed] [Google Scholar]
- Joblin K. N., Johnson A. W., Lappert M. F., Wallis O. C. Coenzyme B-12-dependent reactions. Part IV. Observations on the purification of ethanolamine ammonia-lyase. Biochim Biophys Acta. 1976 Nov 8;452(1):262–270. doi: 10.1016/0005-2744(76)90079-6. [DOI] [PubMed] [Google Scholar]
- Jones A., Faulkner A., Turner J. M. Microbial metabolism of amino alcohols. Metabolism of ethanolamine and 1-aminopropan-2-ol in species of Erwinia and the roles of amino alcohol kinase and amino alcohol o-phosphate phospho-lyase in aldehyde formation. Biochem J. 1973 Aug;134(4):959–968. doi: 10.1042/bj1340959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols via aldehydes. J Gen Microbiol. 1971 Aug;67(3):379–381. doi: 10.1099/00221287-67-3-379. [DOI] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols. 1-Aminopropan-2-ol and ethanolamine metabolism via propionaldehyde and acetaldehyde in a species of Pseudomonas. Biochem J. 1973 May;134(1):167–182. doi: 10.1042/bj1340167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. I. Purification, assay, and properties of the enzyme. J Biol Chem. 1968 Apr 25;243(8):1787–1793. [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. II. Physical and chemical properties and interaction with cobamides and ethanolamine. J Biol Chem. 1968 Apr 25;243(8):1794–1803. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mauck L., Babior B. M. Inactivation of ethanolamine ammonia-lyase by 5,5'-dithiobis(2-nitrobenzoic acid). Further evidence for the involvement of sulfhydryl groups in adenosylcobalamin-dependent rearrangements. Biochim Biophys Acta. 1977 Feb 9;480(2):489–494. doi: 10.1016/0005-2744(77)90041-9. [DOI] [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol. 1976 Jul;95(1):173–176. doi: 10.1099/00221287-95-1-173. [DOI] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


