Abstract
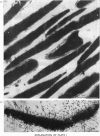
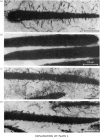
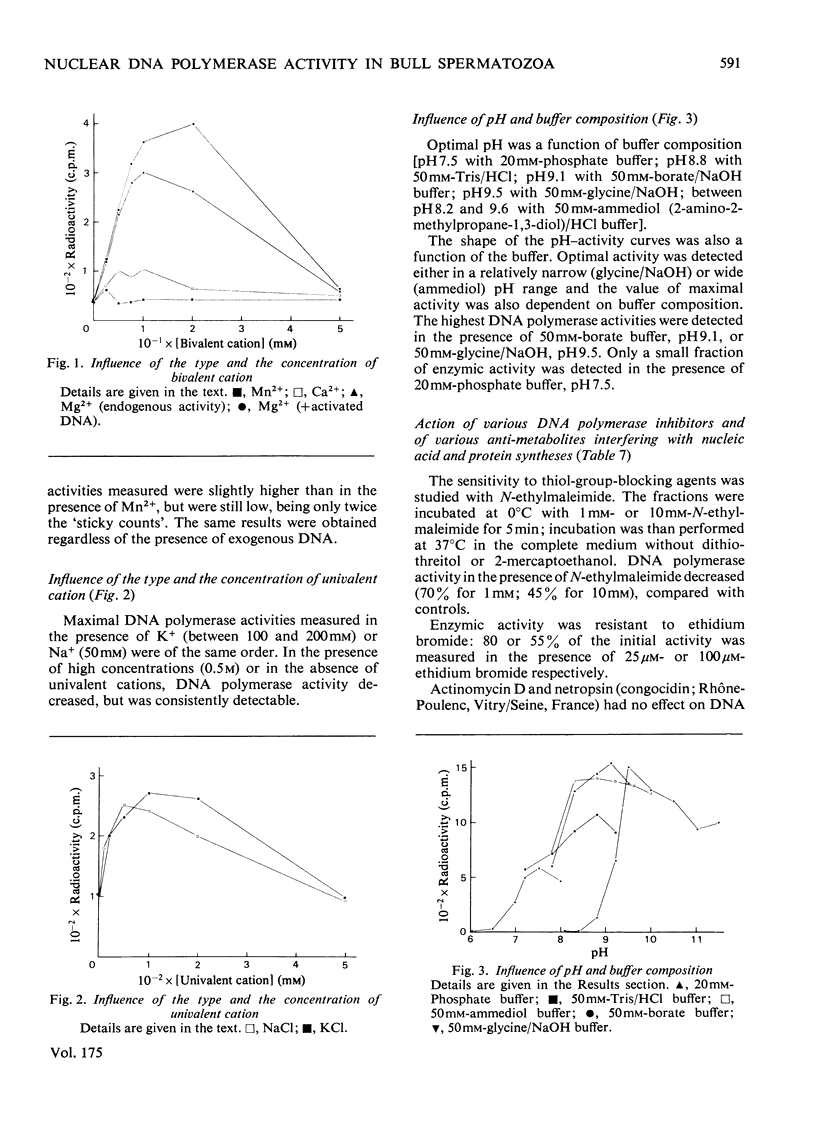
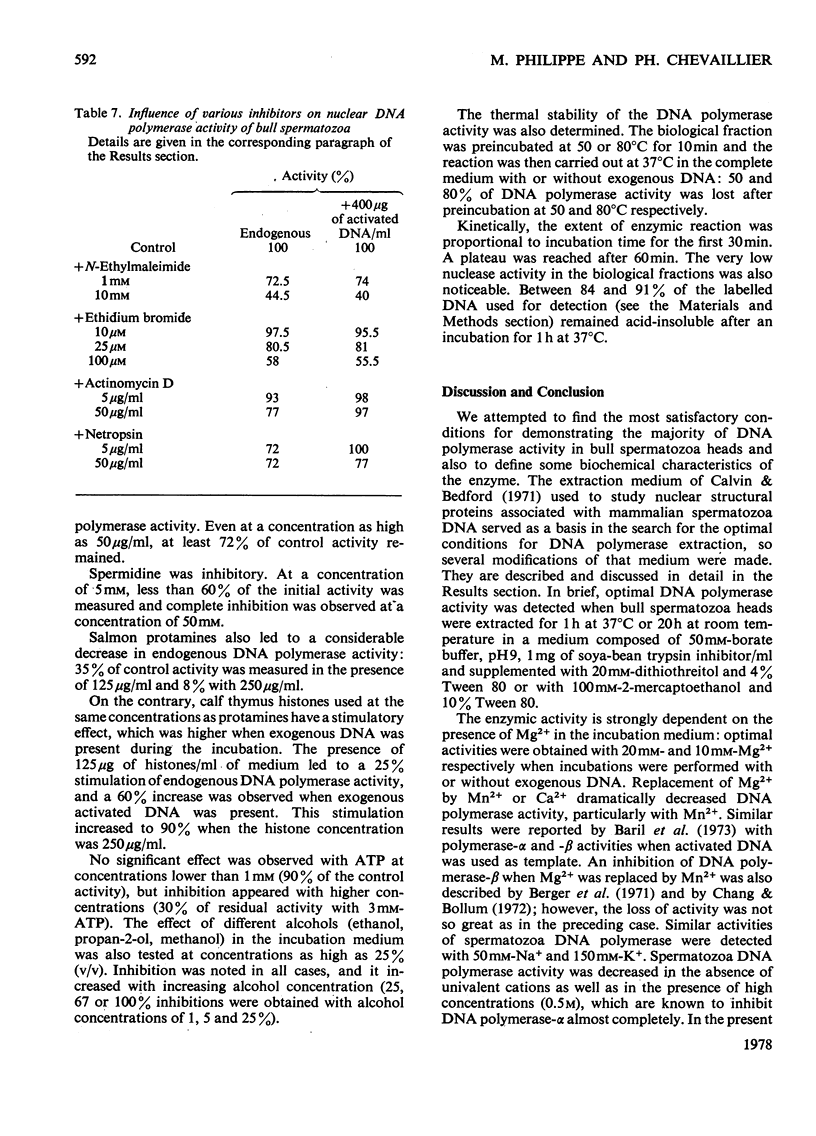
Bull spermatozoa heads were separated from cytoplasmic contaminants, especially mitochondria-rich middle pieces, by centrifugation through 2.4M-sucrose. DNA polymerase activity was demonstrated by incubating nuclear heads for 1 h at 37 degrees C or for 20 h at room temperature in a medium containing detergent and dithiothreitol or 2-mercaptoethanol. Optimal DNA polymerase activity was detected after extraction in a medium containing 50 mM-borate, pH9, 1 mg of soya-bean trypsin inhibitor/ml and supplemented with either 20 mM-dithiothreitol and 4% Tween 80 or 100mM-2-mercaptoethanol and 10% Tween 80. The DNA polymerase reaction was Mg2+-dependent; Mn2+ or Ca2+ could not replace Mg2+ and all four deoxynucleoside triphosphates were required for optimal activity. The polymerase activity was pH-dependent (optimum between 8.2 and 10.5) and was a function of buffer composition and also of pH values. Optimal activity was obtained with 50 mM-Na+ or 150mM-K+ and was partially lowered by N-ethylmaleimide; it was inhibited by spermidine and by salmon protamines, but was greatly stimulated by calf thymus histones. It was also resistant to actinomycin D, netropsin and ethidium bromide. The present results suggest that bull spermatozoa heads contain a beta-type DNA polymerase activity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Henderson M. A., Wood W., Lindsay J. G. Multiple forms of nuclear deoxyribonucleic acid polymerases and their relationship with the soluble enzyme. Biochem J. 1973 Feb;131(2):237–246. doi: 10.1042/bj1310237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril E. F., Jenkins M. D., Brown O. E., Laszlo J., Morris H. P. DNA polymerases I and II in regenerating rat liver and Morris hepatomas. Cancer Res. 1973 Jun;33(6):1187–1193. [PubMed] [Google Scholar]
- Bedford J. M., Bent M. J., Calvin H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil. 1973 Apr;33(1):19–29. doi: 10.1530/jrf.0.0330019. [DOI] [PubMed] [Google Scholar]
- Bendich A., Borenfreund E., Witkin S. S., Beju D., Higgins P. J. Information transfer and sperm uptake by mammalian somatic cells. Prog Nucleic Acid Res Mol Biol. 1976;17:43–75. doi: 10.1016/s0079-6603(08)60065-3. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Calvin H. I., Bedford J. M. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl. 1971 May;13(Suppl):65–75. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase from rabbit bone marrow. Biochemistry. 1972 Mar 28;11(7):1264–1272. doi: 10.1021/bi00757a023. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Phylogeny of DNA polymerase-beta. Science. 1976 Mar 19;191(4232):1183–1185. doi: 10.1126/science.769158. [DOI] [PubMed] [Google Scholar]
- Chevaillier P. H., Philippe M. Acitivé DNA-polymérase nucléaire dans les spermatozoides de souris et évolution de cette activité au cours de la spermatogenèse. Exp Cell Res. 1976 May;99(2):237–244. doi: 10.1016/0014-4827(76)90579-6. [DOI] [PubMed] [Google Scholar]
- Chevaillier P., Philippe M. In situ detection and characterization of DNA polymerase activities in the nucleus of eukaryotic cells. Chromosoma. 1977 Oct 17;63(4):385–399. doi: 10.1007/BF00399497. [DOI] [PubMed] [Google Scholar]
- Chevaillier P., Philippe M. In situ detection of a DNA-polymerase activity in the nuclei of mouse spermatozoa. Chromosoma. 1976 Jan 27;54(1):33–37. doi: 10.1007/BF00331831. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Sung S. C. Solubilization and characterization of particulate form of DNA polymerase from adult rat liver nuclei. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1830–1836. doi: 10.1016/0006-291x(72)90058-7. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Keir H. M. Deoxyribonucleic acid polymerases of BHK-21/C13 cells. Partial purification and characterization of the enzymes. Biochem J. 1975 Feb;145(2):215–224. doi: 10.1042/bj1450215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D. K., Seal G., Loeb L. A. Differential heat sensitivity of mammalian DNA polymerases. Biochem Biophys Res Commun. 1976 May 23;76(2):483–487. doi: 10.1016/0006-291x(77)90750-1. [DOI] [PubMed] [Google Scholar]
- Fansler B. S. Eukaryotic DNA polymerases: their association with the nucleus and relationship to DNA replication. Int Rev Cytol. 1974;Suppl 4:363–415. [PubMed] [Google Scholar]
- Fansler B. S., Loeb L. A. Sea urchin nuclear DNA polymerase. Methods Enzymol. 1974;29:53–70. doi: 10.1016/0076-6879(74)29009-8. [DOI] [PubMed] [Google Scholar]
- Gall W. E., Ohsumi Y. Decondensation of sperm nuclei in vitro. Exp Cell Res. 1976 Oct 15;102(2):349–358. doi: 10.1016/0014-4827(76)90050-1. [DOI] [PubMed] [Google Scholar]
- Hallick L. M., Namba M. Deoxyribonucleic acid synthesis in isolated nuclei from chicken embryo fibroblast cell cultures. Biochemistry. 1974 Jul 16;13(15):3152–3158. doi: 10.1021/bi00712a023. [DOI] [PubMed] [Google Scholar]
- Hecht N. B. A DNA polymerase isolated from bovine spermatozoa. J Reprod Fertil. 1974 Dec;41(2):345–354. doi: 10.1530/jrf.0.0410345. [DOI] [PubMed] [Google Scholar]
- MALKIN H. M. Incorporation of glycine 2-14C and adenine-4,6-14C into the DNA of mature sea urchin sperm. Biochim Biophys Acta. 1953 Dec;12(4):585–586. doi: 10.1016/0006-3002(53)90192-9. [DOI] [PubMed] [Google Scholar]
- Mahi C. A., Yanagimachi R. Induction of nuclear decondensation of mammalian spermatozoa in vitro. J Reprod Fertil. 1975 Aug;44(2):293–296. doi: 10.1530/jrf.0.0440293. [DOI] [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Haemodynamic and coronary vascular responses after beta-adrenoceptor blockade in the anaesthetised dog: a comparison of tolamolol with practolol and propranolol. Biochim Biophys Acta. 1974 Apr 10;340(4):498–508. [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Transformation of sperm histone during formation and maturation of rat spermatozoa. J Biol Chem. 1975 Jan 10;250(1):39–45. [PubMed] [Google Scholar]
- Philippe M., Chevaillier P. Further characterization of a DNA polymerase activity in mouse sperm nuclei. Biochim Biophys Acta. 1976 Oct 4;447(2):188–202. doi: 10.1016/0005-2787(76)90342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe M., Chevaillier P. Presence of two deoxyribonucleic acid polymerases in bull spermatozoa. Biochem J. 1978 Nov 1;175(2):595–600. doi: 10.1042/bj1750595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe M., De Recondo A. M., Chevaillier P. Intranuclear distribution of mouse liver nuclear DNA polymerase. Exp Cell Res. 1976 Mar 15;98(2):424–428. doi: 10.1016/0014-4827(76)90451-1. [DOI] [PubMed] [Google Scholar]
- Poulson R., Krasny J., Zbarsky S. H. Characterisation of nuclear and cytoplasmic DNA polymerases from rat intestinal mucosa. Can J Biochem. 1974 Mar;52(3):162–169. doi: 10.1139/o74-027. [DOI] [PubMed] [Google Scholar]
- Rossignol J. M., Abadiedebat J., Tillit J., de Recondo A. M. ADN polymérases le foie de rat: groupes thiols et activité enzymatique. Biochimie. 1972;54(3):319–324. doi: 10.1016/s0300-9084(72)80210-4. [DOI] [PubMed] [Google Scholar]
- Salisbury G. W., Hart R. G. Gamete aging and its consequences. Biol Reprod Suppl. 1970;2:1–13. [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Stalker D. M., Mosbaugh D. W., Meyer R. R. Novikoff hepatoma deoxyribonucleic acid polymerase. Purification and properties of a homogeneous beta polymerase. Biochemistry. 1976 Jul 13;15(14):3114–3121. doi: 10.1021/bi00659a027. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Ukita T. Purification and further characterization of three DNA polymerases of rat Ascites hepatoma cells. Biochim Biophys Acta. 1974 Jun 27;353(2):146–159. doi: 10.1016/0005-2787(74)90181-6. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Bendich A. DNA synthesizing activity in normal human sperm: location and characterization of the endogenous reaction. Exp Cell Res. 1977 Apr;106(1):47–54. doi: 10.1016/0014-4827(77)90239-7. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Korngold G. C., Bendich A. Ribonuclease-sensitive DNA-synthesizing complex in human sperm heads and seminal fluid. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3295–3299. doi: 10.1073/pnas.72.9.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin B. R., Boison A., Heston W. D., Coffey D. S. Release of DNA template restrictions in rabbit spermatozoa and rat liver nuclei. J Exp Zool. 1976 Aug;197(2):283–288. doi: 10.1002/jez.1401970209. [DOI] [PubMed] [Google Scholar]